Incorporation of deuterium-labelled analogs of isopentenyl diphosphate for the elucidation of the stereochemistry of rubber biosynthesis
Andrew A.
Scholte
and
John C.
Vederas
*
Department of Chemistry, University of Alberta, Edmonton, Alberta, Canada T6G 2G2. E-mail: john.vederas@ualberta.ca; Fax: 00 1 780 492 2134; Tel: 00 1 780 492 5475
First published on 23rd January 2006
Abstract
A series of six deuterium-labelled analogs of isopententyl diphosphate (IPP) was prepared to investigate the detailed stereochemical course of addition of C5 units during rubber biosynthesis in Hevea brasiliensis and Parthenium argentatum. These analogs were incorporated into the cis-polyisoprene chain by rubber transferase in rubber particles, and the stereochemistry was determined by 2H-NMR analysis of the polymer or of levulinic acid derivatives obtained from its ozonolytic degradation. Results indicate that rubber chain elongation occurs with loss of the pro-S hydrogen of IPP, addition of the allylic diphosphate to the si face of IPP and inversion of stereochemistry at the carbon bearing the diphosphate.
Introduction
Rubber is a vital raw material and is used in the production of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 commercial products, including over 400 medical devices.1 Although there are more than 2500 known species of plants that produce rubber, the primary commercial source of natural rubber is the Brazilian rubber tree, Hevea brasiliensis.2 The H. brasilinesis crop consists predominately of plantation-grown clonal trees. This has resulted in a lack of genetic diversity making the crop susceptible to pathogenic attack.3 Therefore, there is considerable interest in developing an alternate commercial source of high quality rubber, for example from the desert shrub, Parthenium argentatum (guayule).4
000 commercial products, including over 400 medical devices.1 Although there are more than 2500 known species of plants that produce rubber, the primary commercial source of natural rubber is the Brazilian rubber tree, Hevea brasiliensis.2 The H. brasilinesis crop consists predominately of plantation-grown clonal trees. This has resulted in a lack of genetic diversity making the crop susceptible to pathogenic attack.3 Therefore, there is considerable interest in developing an alternate commercial source of high quality rubber, for example from the desert shrub, Parthenium argentatum (guayule).4
Natural rubber (cis-1,4-polyisoprene) biosynthesis is catalyzed by rubber transferase (EC 2.5.1.20), a membrane bound cis-prenyltransferase.5,6 This enzyme controls the elongation of the terminal allylic diphosphate of the growing chain by isopentenyl diphosphate 1 (IPP) to form rubber 3 (Scheme 1). The process is usually initiated with a trans allylic diphosphate, for example farnesyl diphosphate 2 (FPP), but all subsequent isoprene units have cis geometry. Rubber transferase also requires a divalent metal cofactor such as Mg2+ or Mn2+ for activity.7
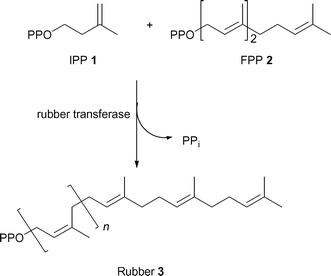 | ||
| Scheme 1 Rubber biosynthesis. | ||
Prenyltransferases, the enzymes that catalyze the formation of linear prenyl chains, have been studied extensively. In 1966, Cornforth et al. delineated the stereochemical outcome of the trans-prenyltransferase, farnesyl diphosphate (FPP) synthase, the enzyme that synthesizes the C15 isoprenoid farnesol skeleton.8,9 By using a series of deuterium labelled (4R)- and (4S)-mevalonic acid substrates, they were able to show that the reaction between IPP and dimethylallyl diphosphate 4 (DMAPP) occurs with overall inversion of stereochemistry of the allylic diphosphate with concomitant elimination of the pro-R proton of IPP to form the trans-alkene of the intermediate geranyl diphosphate 5 (Scheme 2).
 | ||
| Scheme 2 Stereochemical outcome of farnesyl diphosphate synthase. | ||
Cornforth and coworkers also investigated the stereochemistry of natural rubber biosynthesis. Incorporation of labelled (4R) and (4S)-[4-3H1] mevalonate with crude plant homogenates from H. brasiliensis was consistent with the elimination of the pro-S hydrogen.10 However, as it has since been demonstrated that plants biosynthesize IPP through both the cytosolic mevalonic acid pathway and the plastidal methylerythritol 4-phosphate pathway,11,12 the significance of Cornforth's experiments with rubber is less clear.
Studies with undecaprenyl diphosphate (UPP) synthase (a cis-prenyltransferase)13 and heptaprenyl disphosphate synthase (a trans-prenyltransferase)14 led to the axiom that the pro-R hydrogen of IPP is eliminated during the biosynthesis of trans-prenyl chains while the pro-S hydrogen is eliminated for cis-prenyl chains. However, examination of the biosynthesis of polyprenols from the plant Mallotus japonicus illustrated that, contrary to this hypothesis, the pro-R hydrogen is eliminated during the biological formation of the cis-prenyl chains of malloprenols.15
The stereochemical arrangement during the cis C–C bond formation in the biosynthesis of UPP was reported by Ogura and coworkers. Incorporation studies with deuterium-labelled analogs of IPP revealed that reaction occurs on the si face of the double bond in IPP.16,17 Related investigations with the trans-prenyltransferases, heptaprenyl diphosphate synthase and FPP synthase, indicate that the C–C bond formation also occurs at the si face of IPP.17,18 In contrast, studies with FPP synthase from the pea plant, Pisum sativum, indicate that addition occurs on the re face of IPP, which is in disagreement with Cornforth's stereochemical picture of isoprenoid biosynthesis.19,20
Since there are differences between cis-prenyltransferases in the stereochemistry of the hydrogen elimination from IPP, it cannot be stated a priori that rubber transferase will proceed via loss of the pro-R or pro-S proton. Moreover, in order to generate a detailed picture of the stereochemical constraints exerted by rubber transferase, two other stereochemical issues must be addressed, namely which face (re or si) of the double bond in IPP participates in its addition to the growing rubber chain, and whether this attack on the terminal allylic diphosphate proceeds with retention or inversion of stereochemistry. Generally, the displacement of the allylic diphosphate by IPP has been shown to occur with inversion of stereochemistry at the primary allylic carbon.9,21,22 Knowledge of the three dimensional aspects of the rubber transferase reaction can assist understanding of the mechanism of this enzyme that is responsible for production of a major commodity. It also allows comparison to other prenyltransferases and may help in the development of new rubber producing systems, potentially including new rubber crops.
In the present work, we elucidate the cryptic stereochemistry of rubber biosynthesis by synthesis and incorporation of deuterium-labelled analogs of IPP 6–11 (Fig. 1) followed by deuterium NMR analysis of the resulting rubber or its degradation products. Incorporations of compounds 6 and 7 into biosynthetically active rubber particles from H. brasiliensis and P. argentatum can be used to distinguish which face of the alkene participates in its addition to the growing rubber chain, whereas utilisation of 8 and 9 determines the stereochemistry of hydrogen elimination. Incorporation of labelled IPP analogs 10 and 11 verifies the stereochemistry of displacement of the allylic diphosphate during rubber biosynthesis.
 | ||
| Fig. 1 Synthetic target molecules: deuterium analogs of IPP 6–11. | ||
Results and discussion
Substrates 6,237,23 and 824 were prepared as described previously. The synthesis of (S)-[2-2H1]-IPP 9 is analogous to 8 and is shown in Scheme 3. Addition of methylmagnesium bromide to L-glyceraldehyde acetal25,2612 and oxidation of the resulting alcohol with TPAP and NMO provides the methyl ketone 13. Peterson olefination of 13 and deprotection gives 14, which is tosylated in pyridine at 0 °C to afford 15. Ring closure under basic conditions generates the epoxide and treatment with sodium borodeuteride in the presence of boron trifluoride–etherate produces the enantiospecifically deuterium labelled alcohol, which is further reacted with tosyl chloride to yield the tosylate 16. The enantiomeric purity of 16 was determined to be >95% ee.27 Phosphorylation of 16 with tris(tetra-n-butyl)ammonium hydrogen pyrophosphate28 in MeCN yields (S)-[2-2H1]-isopentenyl diphosphate 9.![Synthesis of (S)-[2-2H1]-IPP 9. Reagents and conditions: (i) MeMgBr, Et2O; (ii) TPAP, NMO, 4 Å MS, 39%; (iii) LiCH2Si(Me)3 Et2O, −78 °C, 80%; (iv) HCl, EtOH, 75%; (v) TsCl, pyridine, 71%; (vi) KOH; (vii) NaBD3CN, BF3·OEt2; (viii) TsCl, Et3N, DMAP, 26%; (ix) (Bu4N)3HP2O7, MeCN, 87%.](/image/article/2006/OB/b515750a/b515750a-s3.gif) | ||
| Scheme 3 Synthesis of (S)-[2-2H1]-IPP 9. Reagents and conditions: (i) MeMgBr, Et2O; (ii) TPAP, NMO, 4 Å MS, 39%; (iii) LiCH2Si(Me)3 Et2O, −78 °C, 80%; (iv) HCl, EtOH, 75%; (v) TsCl, pyridine, 71%; (vi) KOH; (vii) NaBD3CN, BF3·OEt2; (viii) TsCl, Et3N, DMAP, 26%; (ix) (Bu4N)3HP2O7, MeCN, 87%. | ||
The chiral diphosphates 10 and 11 can be prepared by phosphorylation of the corresponding chiral alcohols (Scheme 4). Reaction of the Grignard reagent derived from allylic chloride 17 with CO2 affords the allylic acid 18. Reduction of the acid with LiAlD4 provides the di-deuterio alcohol 19. Oxidation of 19 using PCC results in contamination of the desired product 20 by the corresponding conjugated aldehyde. This problem is readily circumvented by the using the mild IBX oxidant.29,30
![Synthesis of (S)- and (R)-[1-2H1]-IPP 10 and 11. Reagents and conditions: (i) Mg, THF, Δ, 2 h; (ii) CO2, 39%; (iii) LiAlD4, Et2O, 70%; (iv) IBX, DMSO; (v) (S)-Alpine borane, THF; (vi) TsCl, Et3N, DMAP, 10% for 21 and 11% for 22 (over 3 steps); (vii) (R)-Alpine borane, THF; (viii) (Bu4N)3HP2O7, MeCN, 87% for 10 and 86% for 11.](/image/article/2006/OB/b515750a/b515750a-s4.gif) | ||
| Scheme 4 Synthesis of (S)- and (R)-[1-2H1]-IPP 10 and 11. Reagents and conditions: (i) Mg, THF, Δ, 2 h; (ii) CO2, 39%; (iii) LiAlD4, Et2O, 70%; (iv) IBX, DMSO; (v) (S)-Alpine borane, THF; (vi) TsCl, Et3N, DMAP, 10% for 21 and 11% for 22 (over 3 steps); (vii) (R)-Alpine borane, THF; (viii) (Bu4N)3HP2O7, MeCN, 87% for 10 and 86% for 11. | ||
Reaction of the labelled aldehyde 20 with (S)-Alpine-Borane® (β-isopinocampheyl-9-boracyclo[3.3.1]nonane)31 gives (1R)-[1-2H1]-3-methylbuten-1-ol. The use of this boron reagent has been recently described for the preparation of chiral deuterium labelled allylic diphosphates by Coates and coworkers.32 Stereochemical analysis using Mosher's ester33 indicates that the alcohol has an ee of 90%. Treatment of this chiral alcohol with tosyl chloride affords the tosylate 21, which is then converted to the desired (1S)-[1-2H1]-IPP 10, by reaction with tris(tetra-n-butyl)ammonium hydrogen pyrophosphate. Analogously, reduction of the aldehyde 20 with (R)-Alpine-Borane® and subsequent reaction with tosyl chloride gives 22, which is then transformed into the enantiomeric target, (1R)-[1-2H1]-IPP 11 (90% ee). The yields of 21 and 22 are not optimized and are low due to volatility of the intermediate aldehyde.
Stereochemistry of hydrogen loss during alkene formation
Initially the stereochemistry of the hydrogen elimination step was investigated. Incorporations of specifically deuterated IPP analogs 8 and 9 should result in retention of deuterium in one case and in loss in the other. This can be easily detected by 2H-NMR analysis of the resulting rubber product. Thus, each of the stereospecifically deuterated IPP's, (R)-[2-2H1]-IPP 8 and (S)-[2-2H1]-IPP 9 were independently incorporated into rubber by incubation with rubber particles from H. brasiliensis and P. argentatum containing rubber transferase. The resulting rubber samples were isolated and analyzed by 2H-NMR spectroscopy.Analyses of the rubber resulting from the incorporations of (S)-[2-2H1]-IPP 9 show no increase in the intensity of the olefinic signals. However, 2H-NMR analysis in CHCl3 of the rubber isolated from incorporation of (R)-[2-2H1]-IPP 8 using either species displays an increase in the intensity of the signal at 5.3 ppm (Fig. 2). These results are consistent with pro-S hydrogen elimination and pro-R hydrogen retention during rubber biosynthesis in both Hevea and Parthenium (Scheme 5). This outcome is also in agreement with elimination of the pro-S hydrogen observed during the biosynthesis of cis-prenyl chains by the bacterial enzyme UPP synthase.13
![2H-NMR (76.5 MHz, CHCl3) spectrum of rubber from incorporation of (R)-[2-2H1]-IPP 8 by rubber particles from H. brasiliensis.](/image/article/2006/OB/b515750a/b515750a-f2.gif) | ||
| Fig. 2 2H-NMR (76.5 MHz, CHCl3) spectrum of rubber from incorporation of (R)-[2-2H1]-IPP 8 by rubber particles from H. brasiliensis. | ||
 | ||
| Scheme 5 Stereochemistry of hydrogen loss during formation of rubber by H. brasiliensis and P. argentatum. | ||
Stereochemical outcome of diphosphate displacement
Analogs of IPP 10 and 11 with stereospecific deuterium labels at the 1-position were used to determine the stereochemistry of the diphosphate displacement (inversion or retention) during rubber biosynthesis. Previously, the stereochemical analysis of farnesyl diphosphate synthase has been accomplished by ozonolysis of the labelled farnesol, obtained after incorporation of 4-deuterio IPPs, to give (R)- or (S)-[3-2H1]-levulinic acid.18 The stereochemistry of the levulinic acid was correlated with known optical rotations values for stereospecifically deuterated levulinic acid.16 However, this analysis relies on quite small optical rotation values to differentiate the enantiomers. Since the enzyme preparations used for the biosynthesis of rubber contain a large amount of existing unlabelled rubber and only produce a small amount of new labelled material, this particular procedure could not be employed for stereochemical analysis.An alternative solution would involve converting the deuterated levulinic acids to their mandelate esters. 2H-NMR spectroscopy could then be used to determine the stereochemistry of the levulinic acid obtained by ozonolytic degradation of rubber isolated from the enzymatic reactions. High resolution 500 MHz NMR analysis of the mandelate ester of levulinic acid in benzene-d6 reveals that all of the diastereotopic hydrogens at C-2 and at C-3 can be differentiated. However, to assign the signals to the pro-R or pro-S hydrogens at each carbon with certainty, synthesis of labelled standards was necessary.
Chiral [2-2H]-levulinic acids appeared available by the hydrolysis of the corresponding nitrile, which in turn, can be accessed by displacement of a tosylate (Scheme 6). Ozonolysis of (1R)-tosylate 21 in CH2Cl2 at −78 °C followed by reduction with zinc and acetic acid gives the methyl ketone 23. Reaction of 23 with sodium cyanide in DMSO generates the volatile nitrile.34 Hydrolysis of the deuterated nitrile with 6 M HCl produces the stereospecifically labelled levulinic acid 25, which can be transformed into ester 27 by coupling with methyl (S)-(+)-mandelate. (2R)-Levulinate 28 can be made in an analogous manner from (1S)-tosylate 22. 2H NMR analysis in C6H6 shows that the deuterium in 27 has a chemical shift of 2.48 ppm, whereas that in 28 is at 2.44 ppm. This difference in chemical shifts is somewhat smaller than expected based on precedents with unfunctionalised alkyl chains studied by Parker35 or having remotely situated carbonyls examined by our group.36,37
 | ||
| Scheme 6 Synthesis of deuterium labelled levulinate standards. Reagents and conditions: (i) O3, −78 °C; (ii) Zn, AcOH, 97% for 23 and 90% for 24; (iii) NaCN, DMSO; (iv) HCl, 34% for both 25 and 26 (over two steps); (v) methyl (S)-(+)-mandelate, DCC, DMAP, 41% for both 27 and 28. | ||
Incorporation of (1S)-[1-2H1] IPP 10 into rubber particles from H. brasiliensis containing active rubber transferase-generated new rubber. Ozonolysis of the rubber in CHCl3 gave levulinic acid, which was then converted into its corresponding mandelate ester. 2H-NMR analysis in C6H6 reveals that the incorporated deuterium has a chemical shift of 2.48 ppm. Correspondingly, incorporation of (1R)-[1-2H1]-IPP 11 ultimately affords a mandelate ester whose deuterium chemical shift in C6H6 is 2.44 ppm. These results indicate that as expected displacement occurs with an overall inversion of stereochemistry at the primary allylic carbon of the growing rubber chain (Scheme 7). Analogous incorporation studies with rubber particles from P. argentatum also show inversion of stereochemistry at the primary allylic carbon.
 | ||
| Scheme 7 Use of 10 and 11 to determine stereochemistry of diphosphate displacement reaction. Reagents and conditions: (i) O3, CH2Cl2; (ii) HCO2H, H2O2; (iii) methyl (S)-(+)-mandelate, DCC, DMAP. | ||
Stereochemical direction of carbon–carbon bond formation
During the biosynthesis of rubber, a new carbon–carbon bond is formed between the double bond of IPP and the allylic carbon of the growing rubber chain. The face of the double bond (si or re) that reacts can potentially be determined by incorporation of Z- and E-[4-2H1]-IPP into rubber by the transferase. Ozonolysis of the resulting rubber would give (3R)- or (3S)-levulinic acid, whose methyl (S)-mandelate esters could be correlated with authentic standards by 2H-NMR analysis.The method described in Scheme 6 might initially seem reasonable for the synthesis of the required standards, but the rapid exchange of the ketone α-protons during hydrolysis of the nitrile precludes this approach. Instead, mild hydration of a terminal acetylene to its corresponding methyl ketone was explored to circumvent this problem for construction of 38 and 39 (Scheme 8).38 Reduction of phenylacetic acid 29 with lithium aluminum deuteride gives the deutero-alcohol 30, which can be oxidized with IBX to [1-2H1]-phenylethanal 31. Reduction with (−)-β-chlorodiisopinocampheylborane39 affords the deuterated alcohol and its subsequent reaction with tosyl chloride and Et3N generates the tosylate 32. Displacement of the primary sulfonate 32 with lithium acetylide–diethylamine complex gives the terminal acetylide 34. Reaction of the acetylide with a stoichiometric amount of mercury (II) acetate followed by reduction of the carbon–mercury bond with sodium borohydride and oxidation of the secondary alcohol provides the desired methyl ketone 36 with no loss of the deuterium label.
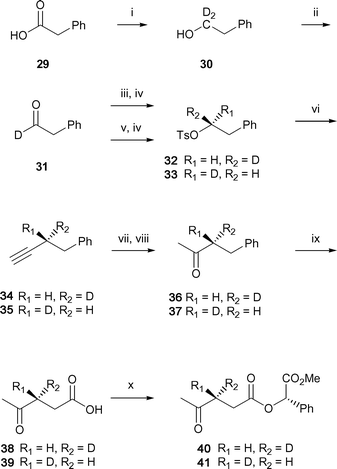 | ||
| Scheme 8 Synthesis of deuterium labelled levulinate standards. Reagents and conditions: (i) LiAlD4, Et2O, 27%; (ii) IBX, DMSO, 91%; (iii) (−)-DIP-Cl, THF; (iv) TsCl, Et3N, DCC, DMAP, 75% for 32 and 86% for 33; (v) (+)-DIP-Cl, THF; (vi) lithium acetylide–diethylamine, DMSO; (vii) Hg(OAc)2, AcOH; (viii) NaBH4, 42% for both 36 and 37 (over two steps); NaIO4, RuCl3, CCl4–MeCN–H2O, 24% for both 38 and 39; (x) methyl (S)-(+)-mandelate, DCC, DMAP, 41% for both 40 and 41. | ||
Oxidation of the phenyl group with ruthenium trichloride and periodic acid gives the desired product, but with the loss of the deuterium label. However, non-acidic oxidation of (3R)-butanone 36 with ruthenium trichloride and sodium periodate40 generates the desired deuterium labelled acid, (3R)-levulinic acid 38, which is converted to 40. Similar reaction conditions allow synthesis of the corresponding ester of (3S)-levulinic acid 41 from (1R)-tosylate 33. 2H-NMR analysis in C6H6 shows that levulinate ester 40 has a deuterium chemical shift of 2.01 ppm, whereas its diastereomer 41 has a chemical shift of 2.21 ppm. The difference in chemical shifts of these diasterotopic hydrogens is unexpectedly large compared to the smaller differences for deuteriums that are closer to the chiral ester group in 27 and 28.
Incorporation of E-[4-2H1]-IPP 6 into rubber particles from H. brasiliensis containing active transferase was followed by ozonolytic degradation to levulinic acid and conversion to the (S)-mandelate ester. 2H-NMR analysis in C6H6 indicates the incorporated deuterium has a chemical of 2.21 ppm, which corresponds to (3S)-[3-2H1]-levulinate 41. Corresponding analysis of the rubber isolated after incorporation of Z-[4-2H1]-IPP 7 shows that the deuterium in the (S)-mandelate ester of levulinic acid has an R configuration These observations demonstrate that si face of the double bond in IPP reacts during the formation of rubber (Scheme 9). Incorporation studies with rubber particles from P. argentatum show the same stereochemical outcome.
 | ||
| Scheme 9 Analysis of stereochemical direction of carbon–carbon bond formation on the IPP double bond. Reagents and conditions: (i) O3, CH2Cl2; (ii) HCO2H, H2O2; (iii) methyl (S)-(+)-mandelate, DCC, DMAP. | ||
Conclusion
In summary, six deuterium-labelled analogs of IPP were prepared to study the stereochemical outcome of rubber biosynthesis in two plant species, H. brasiliensis and P. argentatum. Incorporation of these IPP analogs into rubber particles and 2H-NMR analysis of the rubber and its degradation products indicate that the stereochemistry of rubber transferase in both species is identical and similar to that of the related cis-prenyltransferase, undecaprenyl diphosphate synthase (Scheme 10). Thus, the pro-S hydrogen (Hs) is preferentially cleaved during the polymerization, and si face addition to the allylic diphosphate occurs with overall inversion of stereochemistry. This reveals the 3-dimensional arrangement of substrates within the active site of rubber transferase, and gives a better mechanistic understanding of how rubber is biosynthesized. | ||
| Scheme 10 Stereochemistry of rubber transferase. | ||
Experimental
All chemicals were purchased from Aldrich Chemical company (Madison, WI) or Sigma Chemicals (St. Louis, MO). All solvents, unless otherwise indicated, were HPLC grade and used as such. Tetrahydrofuran (THF) and diethyl ether (Et2O) were distilled over sodium under and an argon atmosphere. Acetonitrile (MeCN), dichloromethane (CH2Cl2), triethylamine and pyridine were distilled from calcium hydride. Methanol (MeOH) and ethanol (EtOH) were distilled over magnesium turnings and a catalytic amount of iodine. Evaporation refers to removal of solvent under reduced pressure on a rotary evaporator. Preparative TLC was performed on glass plates (20 × 20 cm) pre-coated (0.25, 0.5, 1.0 mm) with silica gel (EM Science, Kieselgel 60 F254). Analytical TLC was performed on glass plates (5 × 1.5 cm) pre-coated (0.25 mm) with silica gel (EM Science, Kieselgel 60 F254). Compounds were visualized by exposure to UV light or by staining with a 1% Ce(SO4)2·4H2O 2.5% (NH4)Mo7O24·4H2O in 10% H2SO4 followed by heating on a hot plate. Flash column chromatography was performed with Silicycle silica gel (60, 230–400 mesh).High-performance liquid chromatography (HPLC) was performed on a Beckman System Gold instrument equipped with a model 166 variable wavelength UV detector and an Altex 210A injector with a 500 or 1000 µL sample loop. The columns used were Grace-Vydac. All HPLC solvents were prepared fresh daily and filtered with a Millipore filtration system under vacuum before use.
NMR spectra were recorded on a Varian Inova 600, Inova 400, Inova 300 or Unity 500 spectrometer. For 1H (300, 400, 500 or 600 MHz) values are referenced to 7.24 ppm (CHCl3), to 7.15 ppm (C6H6) and for 13C (100 or 125 MHz) referenced to 77.0 ppm (CDCl3).
Optical rotations were measured on a Perkin-Elmer 241 polarimeter with a micro cell (100 mm path length, 1 mL) and the values are given in 10−1 deg cm2 g−1. IR spectra were recorded on a Nicolet Magna-IR 750 with a Nic-Plan microscope FT-IR spectrometer. Mass spectra were recorded on a Krator AEI MS-50 (HREIMS) and a ZabSpec Isomass VG (HRESMS).
E-[4-2H1]-Isopentenyl diphosphate (6)17,23
A solution of E-[4-2H1]-3-methyl-3-buten-1-yl tosylate (85 mg, 0.35 mmol) in MeCN (10 mL) was treated with tris-n-butylammonium hydrogen diphosphate and the resulting reaction mixture was stirred for 18 h at room temperature. The solvent was evaporated and the residue was dissolved in a minimal volume of IPA–NH4HCO3 (25 mM) 1 : 49. The solution was passed through an ion exchange column containing DOWEX AG 50W X8 (100–200 mesh, 15 equiv.) cation exchange resin (ammonium form). The clear eluent was lyopholized to dryness to give a white solid. 1H NMR analysis indicated that tetra-n-butyl ammonium ion had been exchanged for ammonium ion. The residue was dissolved in 100 mM NH4HCO3 (3 mL) and transferred to a centrifuge tube. A solution of MeCN–IPA 1 : 1 was added, and the contents of the tube were vortexed followed by centrifugation of the suspension for 5 min at 2000 rpm. The supernatant was removed and the process was repeated 3 times. The combined supernatants were concentrated in vacuo and lyopholized. Purification of the crude product using HPLC (Vydac 259VHP reverse phase polymer column; 4.6 × 150 mm; 10% MeCN, 90% 100 mM NH4HCO3 20 min, 10–50% MeCN over 5 min, 50% MeCN 10 min, 50–90% MeCN over 1 min, 90% MeCN 20 min, tR 44.0 min) afforded the title compound 6 as a white solid (88 mg, 85%). IR (µscope) 3141 (b), 3045, 2919, 1405 cm−1; 1H NMR (D2O–ND4OD, 500 MHz) δ the vinylic proton was obscured by solvent, 4.04 (dt, 2H, J = 6.7 Hz, 6.7 Hz CH2OP), 2.38 (t, 2H, J = 6.7 Hz, CH2CH2), 1.76 (s, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); 13C NMR (D2O–ND4OD, 125 MHz) δ 144.6, 111.6 (t, 1JC–D = 23.6 Hz), 64.7 (d, 2JC–P = 5.6 Hz), 38.5, 22.1; 31P NMR (D2O–ND4OD, 162 MHz) δ
−8.80 (m, 1P), −5.10 (m, 1P,); HRMS (ES −ve) calcd for [M − H]− C5H10DO7P2 246.0037, found 246.0035.
CCH3); 13C NMR (D2O–ND4OD, 125 MHz) δ 144.6, 111.6 (t, 1JC–D = 23.6 Hz), 64.7 (d, 2JC–P = 5.6 Hz), 38.5, 22.1; 31P NMR (D2O–ND4OD, 162 MHz) δ
−8.80 (m, 1P), −5.10 (m, 1P,); HRMS (ES −ve) calcd for [M − H]− C5H10DO7P2 246.0037, found 246.0035.
Z-[4-2H1]-Isopentenyl diphosphate (7)17,23
A similar procedure was employed as that described for the preparation of 6. The reaction of Z-[4-2H1]-3-methyl-3-buten-1-yl tosylate (10 mg, 0.041 mmol) in MeCN (10 mL) with tris-n-butylammonium hydrogen diphosphate (111 mg, 0.123 mmol) gave the crude labelled IPP. Purification of the crude product using HPLC (Vydac 259VHP reverse phase polymer column; 4.6 × 150 mm; 10% MeCN, 90% 100 mM NH4HCO3 20 min, 10–50% MeCN over 5 min, 50% MeCN 10 min, 50–90% MeCN over 1 min, 90% MeCN 20 min, tR 44.0 min) afforded the title compound 7 as a white solid (11 mg, 87%). IR (µscope) 3020 (b), 1608, 1559, 1405 cm−1; 1H NMR (D2O–ND4OD, 76.5 MHz) δ 4.84 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, 4.04 (dt, 2H, J = 6.7 Hz, 6.7 Hz, CH2OP), 2.38 (t, 2H, J = 6.7 Hz, CH2CH2), 1.76 (s, 3H, C
CH, 4.04 (dt, 2H, J = 6.7 Hz, 6.7 Hz, CH2OP), 2.38 (t, 2H, J = 6.7 Hz, CH2CH2), 1.76 (s, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); 13C NMR (D2O–ND4OD, 125 MHz) δ 144.3, 111.6 (t, 1JC–D = 23.6 Hz), 66.4 (d, 2JC–P = 5.6 Hz), 38.3, 22.1; 31P NMR (D2O–ND4OD, 162 MHz) δ
−9.04 (m, 1P), −5.01 (m, 1P,); HRMS (ES −ve) calcd for [M − H]− C5H10DO7P2 246.0037, found 246.0037.
CCH3); 13C NMR (D2O–ND4OD, 125 MHz) δ 144.3, 111.6 (t, 1JC–D = 23.6 Hz), 66.4 (d, 2JC–P = 5.6 Hz), 38.3, 22.1; 31P NMR (D2O–ND4OD, 162 MHz) δ
−9.04 (m, 1P), −5.01 (m, 1P,); HRMS (ES −ve) calcd for [M − H]− C5H10DO7P2 246.0037, found 246.0037.
(2R)-[2-2H1]-Isopentenyl diphosphate (8)24,41
A similar procedure was employed as that described for the preparation of 6. The reaction of (R)-[2-2H1]-isopentenyl tosylate (10 mg, 41 µmol) in MeCN (2 mL) with tris-n-butylammonium hydrogen diphosphate (111 mg, 0.123 mmol) gave the crude labelled IPP. Purification of the crude product using HPLC (Vydac 259VHP reverse phase polymer column; 4.6 × 150 mm; 10% MeCN, 90% 100 mM NH4HCO3 20 min, 10–50% MeCN over 5 min, 50% MeCN 10 min, 50–90% MeCN over 1 min, 90% MeCN 20 min, tR 44.0 min) afforded the title compound 8 as a white solid (11 mg, 87%). IR (µscope) 2722 (b), 1685, 1431 cm−1; 1H NMR (D2O–ND4OD, 300 MHz) δ 4.79 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), the other vinylic proton was obscured by solvent, 3.97 (m, 2H, CH2OP), 2.30 (m, 1H, CHDCH2), 1.68 (s, 3H, C
CH), the other vinylic proton was obscured by solvent, 3.97 (m, 2H, CH2OP), 2.30 (m, 1H, CHDCH2), 1.68 (s, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); 13C NMR (D2O–ND4OD, 100 MHz) δ 145.2, 112.8, 67.1 (d, 2JP–P = 5.8 Hz), 39.2 (1JC–D = 22 Hz), 23.1; 31P NMR (D2O–ND4OD, 162 MHz) δ
−9.00 (m, 1P), −5.10 (m, 1P); HRMS (ES −ve) calcd for [M − H]− C5H10DO7P2 246.0037, found 246.0032.
CCH3); 13C NMR (D2O–ND4OD, 100 MHz) δ 145.2, 112.8, 67.1 (d, 2JP–P = 5.8 Hz), 39.2 (1JC–D = 22 Hz), 23.1; 31P NMR (D2O–ND4OD, 162 MHz) δ
−9.00 (m, 1P), −5.10 (m, 1P); HRMS (ES −ve) calcd for [M − H]− C5H10DO7P2 246.0037, found 246.0032.
(2S)-1,2,3-Butanetriol-1,2-acetonide
Methylmagnesium bromide (11.5 mL, 3.0 M solution in Et2O, 34.5 mmol) was added dropwise to a stirring solution of (S)-glyceraldehyde acetonide25,26 (12) (22.9 mmol) in Et2O (15 mL) at 0 °C. The ice bath was removed and stirring was continued at room temperature overnight. The reaction was quenched by pouring the reaction mixture onto ice/saturated NH4Cl solution and the aqueous layer was extracted with Et2O (3 × 15 mL). The combined ethereal extracts were washed with water, brine, dried over Na2SO4 and concentrated in vacuo. Purification of the crude product by flash column chromatography (SiO2, pentanes–Et2O, 1 : 1) afforded the alcohol as a 1 : 1 mixture of diastereomers (1.3 g, 39%). IR (CH2Cl2, cast) 3455, 2986, 2886, 1669, 1456, 1214 cm−1; 1H NMR (CDCl3, 300 MHz) δ 3.88–4.05 (m, 3H, CH2CH, CHOH), 3.70 (dd, 1H, J = 6.2 Hz, 7.9 Hz, CHOC), 2.01 (b, 1H, OH) 1.43 (s, 3H, C(CH3)2), 1.37 (s, 3H, C(CH3)2), 1.16 (d, 3H, J = 6.5 Hz, CHCH3), 1.15 (d, 3H, J = 6.4 Hz, CHCH3); 13C NMR (CDCl3, 125 MHz) δ 109.5, 109.1, 80.4, 79.4, 68.8, 66.8, 66.1, 64.5, 26.7, 26.5, 25.3, 25.2, 18.9, 18.3; HRMS (ES +ve) calcd for [M + Na]+ C7H14O3Na 169.0835, found 169.0837.(3S)-3,4-Dihydroxybutanoneacetonide (13)
A solution of NMO (1.57 g, 13.4 mmol) in CH2Cl2 (20 mL) was treated with MgSO4 and stirred at room temperature for 20 min. After removal of the drying agent by gravity filtration, 4 Å molecular sieves were added followed by a solution of (2S)-1,2,3-butanetriol-1,2-acetonide (1.29 g, 8.95 mmol) in CH2Cl2 (10 mL). The solution was stirred for 15 min prior to the addition of TPAP (50 mg, 0.14 mmol). The reaction was stirred for 6 h at room temperature at which point the solution was filtered through a plug of silica gel to remove the ruthenium catalyst. The eluent was washed with a saturated solution of CuSO4, water, brine and water. The organic layer was dried over MgSO4 and evaporation of the solvent in vacuo gave the ketone 13 as a colorless oil (1.28 g, quantitative). [α]20D = −57.0° (c 1.4, CH2Cl2); IR (CH2Cl2, cast) 2990, 2938, 1720, 1420; cm−1; 1H NMR (CDCl3, 300 MHz) δ 4.39 (dd, 1H, J = 7.7 Hz, 5.6 Hz, CHOC), 4.18 (dd, 1H, J = 8.6 Hz, 7.8 Hz, CH2OC), 3.98 (dd, 1H, J = 8.6 Hz, 5.6 Hz, CH2OC), 2.23 (3H, s, CH3CO), 1.48 (s, 3H, C(CH3)2), 1.38 (s, 3H, C(CH3)2); 13C NMR (CDCl3, 125 MHz) δ 209.2, 111.1, 80.5, 66.5, 26.3, 26.1, 25.1; HRMS (EI) calcd for [M+˙] C7H12O3 144.0786, found 144.0789, 129 (68%), 101 (100%), 83 (18%), 73 (40%), 61 (60%).(2S)-3-(Trimethylsilyl)methyl-1,2,3-butanetriol-1,2-acetonide
To a vigorously stirring solution of acetonide 13 (1.3 g, 9.14 mmol) in Et2O (50 mL) was added (trimethylsilyl)methyllithium (15.5 mL, 1.0 M solution in pentanes, 15.5 mmol) dropwise at −78 °C. The resulting solution was stirred at −78 °C for 1 h at which point a solution of NH4Cl–NaHCO3 (20 mL) was carefully added to quench the reaction. The aqueous layer was separated and extracted with Et2O (3 × 15 mL). The combined ethereal extracts were washed with water and brine, dried over MgSO4, and concentrated in vacuo. Purification of the crude alcohol using flash column chromatography (SiO2, pentanes–Et2O, 10 : 1) afforded the alcohol as a 1 : 1 mixture of diastereomers (2.13 g, 80%). IR (CH2Cl2, cast) 3490, 2986, 2953, 2892, 1456, 1418 cm−1; 1H NMR (CDCl3, 300 MHz) δ 3.81–3.95 (3H, m, CH2OC + CHOC), 1.44 (3H, s, C(CH3)2), 1.38 (3H, s, C(CH3)2), 1.14 (3H, s, CH3COH) 1.07 (1H, d, J = 14.7 Hz, CH2Si), 0.93 (1H, d, J = 14.7 Hz, CH2Si), 0.87 (1H, d, J = 14.7 Hz, CH2Si), 0.72 (1H, d, J = 14.7 Hz, CH2Si), 0.1 (9H, s, Si(CH3)3), 0.07 (9H, s, Si(CH3)3); 13C NMR (CDCl3, 100 MHz) δ 109.4, 109.3, 83.3, 83.2, 72.2, 65.3 (2C), 30.2, 27.4, 26.7, 26.5, 25.5, 25.4, 24.5, 0.6 (3C), 0.5 (3C); HRMS (ES +ve) calcd for [M + Na]+ C11H24O3SiNa 255.1387, found 255.1386.(2R)-3-Methyl-3-butene-1,2-diol (14)
A stirring solution of (2S)-3-(trimethylsilyl)methyl-1,2,3-butanetriol-1,2-acetonide (1.19 g, 5.12 mmol) in EtOH (16 mL) was treated with 3 M HCl (0.5 mL) and the mixture was refluxed for 2 h. After cooling to room temperature the solution was neutralized with the slow addition of solid NaHCO3. The precipitated solids were filtered, washed with EtOH, and the filtrate was concentrated in vacuo. The resulting residue was re-dissolved in MeOH and treated with Et2O (50 mL). The precipitated solids were filtered and the solvent was evaporated in vacuo which afforded the title compound 14 as a colorless oil (390 mg, 75%). [α]20D = −15.7° (c 1.4, CH2Cl2); IR (CH2Cl2, cast) 3373, 3075, 2970, 2879, 1652, 1444; cm−1; 1H NMR (CDCl3, 300 MHz) δ 5.06 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.96 (m, 1H, C
CH2), 4.96 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.18 (dd, 1H, J = 7.2 Hz, 3.4 Hz, CHOH), 3.70 (dd, 1H, J = 11.2 Hz, 3.5 Hz, CH2OH), 3.55 (dd, 1H, J = 11.2 Hz, 7.3 Hz, CH2OH), 2.58 (b, 2H, OH), 1.75 (m, 3H, C
CH2), 4.18 (dd, 1H, J = 7.2 Hz, 3.4 Hz, CHOH), 3.70 (dd, 1H, J = 11.2 Hz, 3.5 Hz, CH2OH), 3.55 (dd, 1H, J = 11.2 Hz, 7.3 Hz, CH2OH), 2.58 (b, 2H, OH), 1.75 (m, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); 13C NMR (CDCl3, 125 MHz) δ 144.1, 112.0, 75.7, 65.2, 18.9; HRMS (EI) calcd for C5H10O2 [M+˙] 102.0681, found 102.0679, 84 (44%), 71 (100%).
CCH3); 13C NMR (CDCl3, 125 MHz) δ 144.1, 112.0, 75.7, 65.2, 18.9; HRMS (EI) calcd for C5H10O2 [M+˙] 102.0681, found 102.0679, 84 (44%), 71 (100%).
(2R)-3-Methyl-3-butene-1,2-diol-1-yl tosylate (15)
To a stirring solution of diol 14 (225 mg, 2.20 mmol) in pyridine (5 mL) at 0 °C was added freshly recrystallized tosyl chloride (462 mg, 2.42 mmol) in pyridine (10 mL) and stirred at 0 °C for 18 h. The solution was then poured onto ice-water and extracted with Et2O (3 × 15 mL). The combined ethereal extracts were washed with 1 M HCl followed by a saturated NaHCO3 solution, water and brine, and dried over MgSO4. Evaporation of the solvent afforded the tosylate 15 as a colorless oil (564 mg, 71%). [α]20D = −13.3° (c 1.2, CH2Cl2); IR (CH2Cl2, cast) 3533, 3069, 2951, 2921, 1652, 1495, 1190 cm−1; 1H NMR (CDCl3, 500 MHz) δ 7.81 (m, 2H, Ar), 7.36 (m, 2H, Ar), 5.06 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.97 (m, 1H, C
CH2), 4.97 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.31 (dd, 1H, J = 7.6 Hz, 3.2 Hz, CHOH), 4.12 (dd, 1H, J = 10.6 Hz, 3.3 Hz, CH2OS), 3.97 (dd, 1H, J = 10.3 Hz, 7.7 Hz, CH2OS), 2.46 (s, 3H, ArCH3), 2.06 (b, 1H, OH) 1.70 (m, 3H, C
CH2), 4.31 (dd, 1H, J = 7.6 Hz, 3.2 Hz, CHOH), 4.12 (dd, 1H, J = 10.6 Hz, 3.3 Hz, CH2OS), 3.97 (dd, 1H, J = 10.3 Hz, 7.7 Hz, CH2OS), 2.46 (s, 3H, ArCH3), 2.06 (b, 1H, OH) 1.70 (m, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); 13C NMR (CDCl3, 100 MHz) δ 145.1, 142.0, 132.8, 129.9 (2C), 128.0 (2C), 113.7, 73.0, 72.4, 21.7, 18.6; HRMS (ES +ve) calcd for [M + Na]+ C12H16O4SNa 279.0662, found 279.0660.
CCH3); 13C NMR (CDCl3, 100 MHz) δ 145.1, 142.0, 132.8, 129.9 (2C), 128.0 (2C), 113.7, 73.0, 72.4, 21.7, 18.6; HRMS (ES +ve) calcd for [M + Na]+ C12H16O4SNa 279.0662, found 279.0660.
(R)-Isopropenyl oxirane42
Finely powdered KOH (1.0 g, 17.8 mmol) was added to tosylate 15 (325 mg, 1.27 mmol) and heated to 60 °C for 1 h. After cooling to room temperature, the residue was dissolved in Et2O and filtered through a mini-pad of Celite® to remove solid impurities. This compound was partially characterized and used without further purification for the subsequent reaction. 1H NMR (CDCl3, 300 MHz) δ 5.18 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.03 (m, 1H, C
CH2), 5.03 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 2.88 (t, 1H, J = 4.2 Hz, CHCH2), 2.73 (dd, 1H, J = 5.3 Hz, 2.7 Hz, CHCH2), 2.47 (m, 1H, CHCH2), 1.64 (s, 3H, C
CH2), 2.88 (t, 1H, J = 4.2 Hz, CHCH2), 2.73 (dd, 1H, J = 5.3 Hz, 2.7 Hz, CHCH2), 2.47 (m, 1H, CHCH2), 1.64 (s, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); 13C NMR (CDCl3, 125 MHz) δ 141.5, 114.6, 54.5, 46.8, 16.2; HRMS (EI) calcd for [M+˙] C5H8O 84.0575, found 84.0563, 69 (35%).
CCH3); 13C NMR (CDCl3, 125 MHz) δ 141.5, 114.6, 54.5, 46.8, 16.2; HRMS (EI) calcd for [M+˙] C5H8O 84.0575, found 84.0563, 69 (35%).
(2S)-[2-2H1]-Isopentenol43
To a stirring solution of (R)-2-isopropenyl oxirane, sodium cyanoborodeuteride (167 mg, 2.54 mmol) and a small amount bromocresol green (5 mg) in Et2O (5 ml) was added dropwise a BF3–etherate solution until the color of the solution was observed to be yellow. The resulting mixture was stirred at room temperature for 5 h and additional amounts of BF3–etherate were added periodically to keep the solution acidic. The solution was diluted with brine and extracted with Et2O (3 × 15 mL). The combined ethereal extracts were dried over MgSO4 and evaporation of the solvent in vacuo at 0 °C gave the deuterio alcohol as a colorless liquid. The volatile alcohol was partially characterized and used in the next step without further purification. 1H NMR (CDCl3, 500 MHz) δ 4.87 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.79 (m, 1H, C
CH2), 4.79 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 3.72 (m, 2H, CH2OH), 2.29 (m, 1H, CHDCH2), 1.76 (m, 3H, C
CH2), 3.72 (m, 2H, CH2OH), 2.29 (m, 1H, CHDCH2), 1.76 (m, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3).
CCH3).
(2S)-[2-2H1]-Isopentenyl tosylate (16)41
A stirring solution of (2S)-[2-2H1]-isopentenol and tosyl chloride (67 mg, 1.91 mmol) in CH2Cl2 (5 mL) was treated with Et3N (0.27 mL, 1.91 mmol) and DMAP (10 mg (136 mg, 1.11 mmol). The resulting mixture was stirred at room temperature for 12 h, at which time Et2O (50 mL) was added. Precipitated solids were removed by filtration, and the filtrate was concentrated in vacuo. Purification of the crude tosylate by flash column chromatography (SiO2, hexane–EtOAc, 10 : 1) afforded the tosylate 16 as an oil (80 mg, 26% for three steps). [α]20D = −0.4° (c 1.0, CH2Cl2); IR (CH2Cl2, cast) 3078, 2971, 1652, 1495, 1177 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.79 (m, 2H, Ar), 7.34 (m, 2H, Ar), 4.79 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH2), 4.68 (m, 1H, C
CCH2), 4.68 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH2), 4.12 (m, 2H, CH2OS), 2.45 (s, 3H, ArCH3), 2.34 (m, 1H, CHDCH2), 1.66 (m, 3H, C
CCH2), 4.12 (m, 2H, CH2OS), 2.45 (s, 3H, ArCH3), 2.34 (m, 1H, CHDCH2), 1.66 (m, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); 2H NMR (CHCl3, 61.4 MHz) δ 2.36; 13C NMR (CDCl3, 125 MHz) δ 144.8, 140.1. 133.2, 129.8, 127.9, 113.2, 66.8, 36.4 (t, J = 19.5 Hz), 22.3, 21.7; HRMS (EI) calcd for [M+˙] C12H15DO3S 241.0883, found 241.0877, 155 (52%), 91 (79%).
CCH3); 2H NMR (CHCl3, 61.4 MHz) δ 2.36; 13C NMR (CDCl3, 125 MHz) δ 144.8, 140.1. 133.2, 129.8, 127.9, 113.2, 66.8, 36.4 (t, J = 19.5 Hz), 22.3, 21.7; HRMS (EI) calcd for [M+˙] C12H15DO3S 241.0883, found 241.0877, 155 (52%), 91 (79%).
(2S)-[2-2H1]-Isopentenyl diphosphate (9)41
A similar procedure was employed as that described for the preparation of 6. The reaction of (2S)-[2-2H1]-isopentenyl tosylate (16) (10 mg, 0.041 mmol) in MeCN (2 mL) with tris-n-butylammonium hydrogen diphosphate gave the crude labelled IPP. The reaction was worked up as previously described. Purification of the crude product using HPLC (Vydac 259VHP reverse phase polymer column; 4.6 × 150 mm; 10% MeCN, 90% 100 mM NH4HCO3 20 min, 10–50% MeCN over 5 min, 50% MeCN 10 min, 50–90% MeCN over 1 min, 90% MeCN 20 min, tR 44.0 min) afforded the title compound 9 as a white solid (11 mg, 87%). IR (µscope) 2707 (b), 1685, 1430 cm−1; 1H NMR (D2O–ND4OD, 400 MHz) δ 4.99 (m, 2H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 4.18 (m, 2H, CH2OP), 2.53 (m, 1H, CHDCH2), 1.91 (s, 3H, C
CH), 4.18 (m, 2H, CH2OP), 2.53 (m, 1H, CHDCH2), 1.91 (s, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); 13C NMR (D2O–ND4OD, 100 MHz) δ 145.2, 112.8, 67.1 (d, 2JP–P = 5.8 Hz), 39.2 (1JC–D = 22 Hz), 23.1; 31P NMR (D2O–ND4OD, 162 MHz) δ
−9.05 (m, 1P), −5.05 (m, 1P); HRMS (ES −ve) calcd for [M − H]− C5H10DO7P2 246.0037, found 246.0036.
CCH3); 13C NMR (D2O–ND4OD, 100 MHz) δ 145.2, 112.8, 67.1 (d, 2JP–P = 5.8 Hz), 39.2 (1JC–D = 22 Hz), 23.1; 31P NMR (D2O–ND4OD, 162 MHz) δ
−9.05 (m, 1P), −5.05 (m, 1P); HRMS (ES −ve) calcd for [M − H]− C5H10DO7P2 246.0037, found 246.0036.
3-Methyl-3-butenoic acid (18)44
In a flame dried, argon flushed round-bottom flask was added freshly ground magnesium turnings (4.98 g, 205 mmol) and THF (75 mL). To this suspension, a solution of 3-chloro-2-methyl-propene (17) (10.0 g, 221 mmol) in THF (25 mL) was then added dropwise, followed by a crystal of iodine. After the addition was complete the reaction mixture was refluxed for 2 h and cooled to room temperature. The reaction was stirred for 30 min and then cooled to −78 °C with a dry ice–acetone bath. At this point, CO2 gas was bubbled though the solution for 1 h and then the temperature was slowly allowed to increase to 0 °C by removal of the ice bath. Once the reaction reached 0 °C, it was cooled back down to −78 °C with bubbling of CO2 through solution for 10 min and then the reaction mixture was allowed to warm up to 10 °C. The solution was basified to pH 10 with cold 2 M NaOH and washed with Et2O (3 × 50 mL). The aqueous layer was then acidified with cold 4 M HCl to pH 2 and extracted with Et2O (3 × 75 mL). The combined ethereal extracts were washed with brine, dried over MgSO4 and concentrated in vacuo to give the acid 18 as a colorless oil (4.0 g, 39%). IR (CH2Cl2, cast) 3083, 2978, 1711, 1651, 1413 cm−1; IR (CH2Cl2, cast) 3083, 2978, 1711, 1651, 1413 cm−1; 1H NMR (CDCl3, 300 MHz) δ 10.0 (b, 1H, CO2H), 4.97 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.90 (m, 1H, C
CH2), 4.90 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 3.09 (d, 2H, J = 1.0 Hz, CH2), 1.85 (m, 3H, C
CH2), 3.09 (d, 2H, J = 1.0 Hz, CH2), 1.85 (m, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); 13C NMR (CDCl3, 100 MHz) δ 177.7, 137.9, 115.3, 43.1, 22.4; HRMS (EI) calcd for [M+˙] C5H8O2 100.0524, found 100.0521, 85 (17%), 82 (22%), 72 (32%), 59 (37%), 55 (76%).
CCH3); 13C NMR (CDCl3, 100 MHz) δ 177.7, 137.9, 115.3, 43.1, 22.4; HRMS (EI) calcd for [M+˙] C5H8O2 100.0524, found 100.0521, 85 (17%), 82 (22%), 72 (32%), 59 (37%), 55 (76%).
[1,1-2H2]-3-Methyl-3-buten-1-ol (19)22
A solution of lithium aluminum deuteride (5.0 ml, 1.0 M solution in Et2O, 5.0 mmol) was added dropwise to a stirring solution of 3-methyl-3-butenoic acid (18) (1.0 g, 10.0 mmol) in Et2O (10 mL) at 0 °C. The ice bath was removed and stirring was continued for 3 h at room temperature. The reaction mixture was then cooled to 0 °C and quenched with slow addition of solid Na2SO4·10H2O and stirred for 30 min. The precipitated aluminum salts were removed by filtration and the filtrate was concentrated in vacuo to give the alcohol 19 as a colorless liquid (0.88 g, 70%). IR (CH2Cl2, cast) 3346, 3076, 2917, 2849, 2208, 1651, 1455 cm−1; 1H NMR (CDCl3, 300 MHz) δ 4.86 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH2), 4.78 (m, 1H, C
CCH2), 4.78 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH2), 2.28 (s, 2H, CH2CD2), 1.75 (m, 3H, C
CCH2), 2.28 (s, 2H, CH2CD2), 1.75 (m, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); 2H NMR (CHCl3, 61.4 MHz) δ 3.68; 13C NMR (CDCl3, 100 MHz) δ 144.2, 112.7, 59.5 (qn, 1JC–D = 22 Hz), 40.7, 22.2; HRMS (EI) calcd for [M+˙] C5H8D2O 88.0857, found 88.0854, 70 (98%), 56 (100%).
CCH3); 2H NMR (CHCl3, 61.4 MHz) δ 3.68; 13C NMR (CDCl3, 100 MHz) δ 144.2, 112.7, 59.5 (qn, 1JC–D = 22 Hz), 40.7, 22.2; HRMS (EI) calcd for [M+˙] C5H8D2O 88.0857, found 88.0854, 70 (98%), 56 (100%).
[1-2H1]-3-Methylbut-3-en-1-al (20)22
To a stirring solution of IBX (4.88 g, 17.4 mmol) in DMSO (40 mL) was added [1,1-2H2]-3-methyl-3-buten-1-ol (19) (500 mg, 5.67 mmol) and stirred at room temperature for 18 h. The solution was cooled to 0 °C and then H2O (5 mL) was added the resulting mixture was stirred for an additional 5 min at 0 °C. The mixture was filtered through a pad of Celite® and the filtrate was extracted exhaustively with Et2O. The combined ethereal extracts were washed with brine, dried over MgSO4 and concentrated in vacuo to afford the aldehyde 20 as colorless oil. The product was used without further purification as the aldehyde is very unstable to heat or base, and isomerization to the conjugated aldehyde is observed during storage. IR (CH2Cl2, cast) 3077, 2953, 1732, 1651, 1436 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.00 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.84 (m, 1H, C
CH2), 4.84 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 3.06 (s, 2H, CH2CDO), 1.78 (m, 3H, C
CH2), 3.06 (s, 2H, CH2CDO), 1.78 (m, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3).
CCH3).
(1R)-[1-2H1]-Isopentenyl tosylate (21)
To a stirring solution of [1-2H1]-3-methylbut-3-en-1-al (20) (5.67 mmol) in Et2O at 0 °C was added a solution of (S)-Alpine borane (13.6 mL, 0.5 M solution in THF, 6.80 mmol) dropwise over 20 min. After addition, the reaction was warmed to room temperature and stirred for 18 h. Acetaldehyde (0.38 mL, 0.79 mmol) was added and stirred for 5 min at which time the volatiles were removed by evaporation (40 °C, 0.05 mm Hg). The residue was dissolved in Et2O and was treated with ethanolamine (0.41 mL, 6.80 mmol) and the solids were removed by filtration. The filtrate was concentrated in vacuo and purification of the crude product by flash column chromatography (SiO2, pentanes–Et2O, 4 : 1) afforded the alcohol. Tosyl chloride (1.19 g, 6.23 mmol), Et3N (0.96 mL, 6.85 mmol) and DMAP (10 mg, 0.08 mmol) was added to a solution of the crude alcohol in CH2Cl2 (10 mL). The resulting reaction mixture was stirred at room temperature for 18 h at which time it was diluted with water (10 mL) and extracted with CH2Cl2. The combined organic extracts were washed with water and brine, dried over MgSO4 and concentrated in vacuo. The crude tosylate was purified using flash column chromatography (SiO2, hexane–EtOAc, 10 : 1) to afford the title compound 21 as a colorless oil (131 mg, 10% for three steps). [α]20D = +0.9° (c 1.5, CH2Cl2); IR (CH2Cl2, cast) 2927, 1649, 1364, 1177 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.76 (m, 2H, Ar), 7.32 (m, 2H, Ar), 4.76 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.65 (m, 1H, C
CH2), 4.65 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.11 (tt, 1H, J = 6.9 Hz, 1.2 Hz CHDOS), 2.45 (s, 3H, ArCH3), 2.32 (d, 2H, J = 6.9 Hz, CH2CHD), 1.63 (m, 3H, C
CH2), 4.11 (tt, 1H, J = 6.9 Hz, 1.2 Hz CHDOS), 2.45 (s, 3H, ArCH3), 2.32 (d, 2H, J = 6.9 Hz, CH2CHD), 1.63 (m, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); 2H NMR (CHCl3, 76.5 MHz) δ 4.11; 13C NMR (CDCl3, 100 MHz) δ 144.7, 140.2, 133.3, 129.9 (2C), 128.0 (2C), 113.1, 68.3 (t, J = 22.9 Hz), 36.7, 22.4, 21.7; HRMS (ES +ve) calcd for [M + Na]+ C12H15DO3SNa 264.0775, found 264.0773.
CCH3); 2H NMR (CHCl3, 76.5 MHz) δ 4.11; 13C NMR (CDCl3, 100 MHz) δ 144.7, 140.2, 133.3, 129.9 (2C), 128.0 (2C), 113.1, 68.3 (t, J = 22.9 Hz), 36.7, 22.4, 21.7; HRMS (ES +ve) calcd for [M + Na]+ C12H15DO3SNa 264.0775, found 264.0773.
(1S)-[1-2H1]-Isopentenyl tosylate (22)
A similar procedure was employed as that described for the preparation of 21. Substitution of (R)-Alpine borane for the (S)-Alpine borane in the procedure used to prepare the enantiomeric tosylate 21 gave the desired compound 22. [α]20D = −1.8° (c 0.5, CH2Cl2); IR (CH2Cl2, cast) 3078, 2926, 2200, 1653, 1598, 1365 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.76 (2H, m, Ar), 7.32 (2H, m, Ar), 4.76 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.65 (m, 1H, C
CH2), 4.65 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.11 (tt, 1H, J = 7.0 Hz, 1.2 Hz, CHDOS), 2.45 (s, 3H, ArCH3), 2.32 (d, 2H, J = 6.8 Hz, CHDCH2), 1.63 (m, 3H, C
CH2), 4.11 (tt, 1H, J = 7.0 Hz, 1.2 Hz, CHDOS), 2.45 (s, 3H, ArCH3), 2.32 (d, 2H, J = 6.8 Hz, CHDCH2), 1.63 (m, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); 2H NMR (CHCl3, 76.5 MHz) δ 4.11; 13C NMR (CDCl3, 100 MHz) δ 144.8, 140.1, 133.3, 129.9 (2C), 128.0 (2C), 133.1, 68.3 (t, 1JC–D = 23.0 Hz), 36.7, 22.4, 21.7; HRMS (ES +ve) calcd for [M + Na]+ C12H15DO3SNa 264.0775, found 264.0773.
CCH3); 2H NMR (CHCl3, 76.5 MHz) δ 4.11; 13C NMR (CDCl3, 100 MHz) δ 144.8, 140.1, 133.3, 129.9 (2C), 128.0 (2C), 133.1, 68.3 (t, 1JC–D = 23.0 Hz), 36.7, 22.4, 21.7; HRMS (ES +ve) calcd for [M + Na]+ C12H15DO3SNa 264.0775, found 264.0773.
(1S)-[1-2H1]-Isopentenyl diphosphate (10)
A similar procedure was employed as that described for the preparation of 6. The reaction of (1R)-[1-2H1]-isopentenyl tosylate (21) (10.5 mg, 44 µmol) in MeCN (2 mL) with tris-n-butylammonium hydrogen diphosphate (118 mg, 0.131 mmol) gave the crude labelled IPP. Purification of the crude product using HPLC (Vydac 259VHP reverse phase polymer column; 4.6 × 150 mm; 10% MeCN, 90% 100 mM NH4HCO3 20 min, 10–50% MeCN over 5 min, 50% MeCN 10 min, 50–90% MeCN over 1 min, 90% MeCN 20 min, tR 44.0 min) afforded the title compound 10 as a white solid (10 mg, 87%). IR (µscope) 2722 (b), 1685, 1431 cm−1; 1H NMR (D2O–ND4OD, 600 MHz) δ 5.10 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.08 (m, 1H, C
CH), 5.08 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) 4.27 (dt, 1H, J = 6.8 Hz, 6.8 Hz, CHDOP), 2.62 (d, 2H, J = 6.8 Hz, CH2CHD), 2.02 (m, 3H, C
CH) 4.27 (dt, 1H, J = 6.8 Hz, 6.8 Hz, CHDOP), 2.62 (d, 2H, J = 6.8 Hz, CH2CHD), 2.02 (m, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); 13C NMR (D2O–ND4OD, 125 MHz) δ 144.6, 112.2, 64.4 (dt, 1JC–D = 22.9 Hz, 2JC–P = 5.5 Hz), 38.4, 22.3; 31P NMR (D2O–ND4OD, 162 MHz) δ
−9.00 (m, 1P), −5.10 (m, 1P); HRMS (ES −ve) calcd for [M − H]− C5H10DO7P2 246.0037, found 246.0035.
CCH3); 13C NMR (D2O–ND4OD, 125 MHz) δ 144.6, 112.2, 64.4 (dt, 1JC–D = 22.9 Hz, 2JC–P = 5.5 Hz), 38.4, 22.3; 31P NMR (D2O–ND4OD, 162 MHz) δ
−9.00 (m, 1P), −5.10 (m, 1P); HRMS (ES −ve) calcd for [M − H]− C5H10DO7P2 246.0037, found 246.0035.
(1R)-[1-2H1]-Isopentenyl diphosphate (11)
A similar procedure was employed as that described for the preparation of 6. The reaction of (1S)-[2-2H]-isopentenyl tosylate (22) (11.0 mg, 0.044 mmol) in MeCN (2 mL) with tris-n-butylammonium hydrogen diphosphate (123 mg, 0.137 mmol) gave the crude labelled IPP. Purification of the crude product using HPLC HPLC (Vydac 259VHP reverse phase polymer; 4.6 × 150 mm; 10% MeCN, 90% 100 mM NH4HCO3 20 min, 10–50% MeCN over 5 min, 50% MeCN 10 min, 50–90% MeCN over 1 min, 90% MeCN 20 min, tR 44.0 min) afforded the title compound 11 as a white solid (12 mg, 86%). IR (µscope) 2846 (b), 1652, 1436 cm−1; 1H NMR (D2O–ND4OD, 500 MHz) δ 5.10 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) 5.08 (m, 1H, C
CH) 5.08 (m, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 4.26 (m, 1H, CHDOP), 2.61 (d, 2H, J = 6.8 Hz, CH2CHD), 2.00 (m, 3H, C
CH), 4.26 (m, 1H, CHDOP), 2.61 (d, 2H, J = 6.8 Hz, CH2CHD), 2.00 (m, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3); δ13C NMR (D2O–ND4OD, 125 MHz) δ 144.6, 112.2, 64.4 (dt, 1JC–D = 22.9 Hz, 2JC–P = 5.5 Hz), 38.4, 22.6; 31P NMR (D2O–ND4OD, 162 MHz) δ
−9.00 (m, 1P), −5.10 (m, 1P); HRMS (ES −ve) calcd for [M − H]− C5H10DO7P2 246.0037, found 246.0038.
CCH3); δ13C NMR (D2O–ND4OD, 125 MHz) δ 144.6, 112.2, 64.4 (dt, 1JC–D = 22.9 Hz, 2JC–P = 5.5 Hz), 38.4, 22.6; 31P NMR (D2O–ND4OD, 162 MHz) δ
−9.00 (m, 1P), −5.10 (m, 1P); HRMS (ES −ve) calcd for [M − H]− C5H10DO7P2 246.0037, found 246.0038.
(4R)-[4-2H1]-2-butanone-4-yl tosylate (23)
(1R)-[1-2H1]-Isopentenyl tosylate (21) (200 mg, 0.832 mmol) was dissolved in CH2Cl2 (10 mL) and cooled to −78 °C in a dry ice–acetone bath. To this solution was bubbled O3 for 20 min at which time the solution was purple in color. Excess O3 was purged by bubbling O2 through the solution for 5 min. The solution was then warmed to room temperature and zinc (109 mg, 1.67 mmol) was added, followed by acetic acid (0.26 mL, 4.50 mmol) and stirred at room temperature for 2 h. The reaction mixture was diluted with H2O and extracted with Et2O. The combined ethereal extracts were washed with a saturated solution of NaHCO3, water and brine, dried over MgSO4 and concentrated in vacuo to give the tosylate 23 (195 mg, 97%). For unlabelled material: IR (CH2Cl2, cast) 2921, 1719, 1359, 1176 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.77 (m, 2H, Ar), 7.33 (m, 2H, Ar), 4.24 (t, 2H, J = 6.4 Hz, CH2OS), 2.81 (t, 2H, J = 6.4 Hz, CH2CO), 2.43 (s, 3H, ArCH3) 2.13 (s, 3H, CH3CO); 13C NMR (CDCl3, 125 MHz) δ 204.3, 144.9, 132.7, 129.9 (2C), 128.0 (2C), 64.9, 42.2, 30.3, 21.7; HRMS (EI) calcd for [M+˙] C11H14O4S 242.0613, found 242.0619, 214 (5%), 172 (100%), 155 (27%), 108 (42%), 91 (100%). The (4R)-[4-2H1]-2-butanone-4-yl-tosylate showed identical chromatographic properties and displayed similar spectral properties except for the following: 1H NMR (CDCl3, 300 MHz) δ 7.77 (m, 2H, Ar), 7.33 (m, 2H, Ar), 4.24 (m, 1H, CHDOS), 2.82 (d, 2H, J = 6.3 Hz, CH2CHD), 2.43 (s, 3H, ArCH3) 2.13 (s, 3H, CH3CO); 2H NMR (C6H6, 76.5 MHz) δ 4.24.(4S)-[4-2H1]-2-Butanone-4-yl tosylate (24)
Substitution of (1S)-[1-2H]-isopentenyl tosylate (22) for (1R)-[1-2H]-isopentenyl tosylate (21) in the procedure used to prepare the enantiomeric tosylate 23 gave the title compound 24. Chromatographic properties and spectral data were identical.(2S)-[2-2H1]-Levulinic acid (25)
A stirring solution of (4R)-[4-2H1]-2-butanone-4-yl tosylate (23) (92 mg, 0.38 mmol) in DMSO (5 mL) was treated with sodium cyanide (56 mg, 1.14 mmol) and stirred at 60 °C for 12 h. The reaction mixture was cooled to room temperature and extracted with CH2Cl2. The combined organic extracts were washed with brine, dried over MgSO4 and concentrated in vacuo. To the crude nitrile was added 6 M HCl (1.5 mL, 1.5 mmol) and heated to reflux for 5 h. The reaction mixture was cooled to room temperature and extracted with CH2Cl2. The combined organic extracts were washed with brine, dried over MgSO4 and concentrated in vacuo to give the crude acid 25 (15 mg, 34%). This compound was used without further purification for the preparation of (S)-(+)-(methoxycarbonyl)benzyl (2S)-[2-2H1]-levulinate (27). For unlabelled material: IR (CH2Cl2, cast) 3111, 1716, 1402, 1369, 1165 cm−1; 1H NMR (CDCl3, 300 MHz) δ 2.73 (m, 2H, CH2CO), 2.61 (m, 2H, CH2COOH), 2.17 (s, 3H, CH3CO); 13C NMR (CDCl3, 100 MHz) δ 206.9, 178.4, 37.7, 29.7, 27.8; HRMS (EI) calcd for [M+˙] C5H8O3 116.0474, found 116.0472, 101 (22%), 73 (47%), 56 (100%). The (2S)-[2-2H1]-levulinic acid (25) showed identical chromatographic and spectral properties except for the following: 2H NMR (C6H6, 61.4 MHz) δ 2.61.(2R)-[2-2H1]-Levulinic acid (26)
Substitution of (4S)-[4-2H1]-2-butanone-4-yl tosylate (24) into the described procedure used to prepare the enantiomeric acid 25 gave the title compound 26. Chromatographic properties and spectral data were identical to those of 25.(S)-(+)-(Methoxycarbonyl)benzyl (2S)-[2-2H1]-levulinate (27)
To a stirring solution of (2S)-[2-2H1]-levulinic acid (25) (15 mg, 0.13 mmol) in CH2Cl2 (5 mL) was added (S)-(+)-mandelic acid methyl ester (24 mg, 0.14 mmol), DCC (32 mg, 0.16 mmol) and DMAP (2 mg, 0.02 mmol). The resulting reaction mixture was stirred at room temperature for 18 h and filtered through a sintered glass funnel to remove solid impurities. The crude ester was purified by flash column (SiO2, hexane–EtOAc, 4 : 1) to afford the title compound 27 (14 mg, 41%) as a colorless oil. For unlabelled material: IR (CH2Cl2, cast) 3034, 2954, 1745, 1719, 1587, 1364, 1152 cm−1; 1H NMR (C6D6, 300 MHz) δ 7.42 (m, 2H, Ar), 7.04 (m, 3H, Ar), 6.05 (s, 1H, CHCO2Me), 3.18 (s, 3H, OCH3), 2.50 (m, 2H, J = 7.0 Hz, CH2CO2R), 2.21 (td, 1H, J = 6.3 Hz, 18.2 Hz, CH2CO), 2.01 (td, 1H, J = 6.3 Hz, 18.2 Hz, CH2CO), 2.01 (s, 3H, CH3CO); 13C NMR (CDCl3, 125 MHz) δ 206.1, 172.1, 169.2, 133.7, 129.3, 128.8 (2C), 127.6 (2C), 74.6, 52.6, 37.8, 29.8, 27.8; HRMS (EI) calcd for [M+˙] C14H16O5 264.0998, found 264.0989, 232 (5%), 205 (4%), 166 (7%), 149 (6%), 121 (10%), 99 (100%). The (S)-(+)-(methoxycarbonyl)benzyl (2S)-[2-2H1]-levulinate (27) showed identical chromatographic properties and displayed similar spectral properties except for the following: 2H NMR (C6H6, 76.5 MHz) δ 2.48.(S)-(+)-(Methoxycarbonyl)benzyl (2R)-[2-2H1]-levulinate (28)
A procedure similar to that used for the preparation of (S)-(+)-(methoxycarbonyl)benzyl (2S)-[3-2H1]-levulinate (27) was employed except that (2R)-[2-2H1]-levulinic acid (26) was used as the starting material. Spectral data were similar to those of 27 except for 2H NMR (C6H6, 76.5 MHz) δ 2.44[1,1-2H2]-2-Phenylethanol (30)45
A similar procedure was employed as that described for the preparation of 19. The reaction of phenylacetic acid (29) (5.0 g, 36.7 mmol) with a solution of lithium aluminum deuteride (12.3 ml of 1.0 M solution in Et2O) afforded the alcohol 30 (1.25 g, 27%) IR (CH2Cl2, cast) 3311, 3086, 3062, 3028, 2932, 2213, 1603, 1496 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.36–7.21 (5H, m, Ar), 2.87 (2H, s, CH2); 2H NMR (CHCl3, 76.5 MHz) δ 3.86; 13C NMR (CDCl3, 100 MHz) δ 138.4, 129.0 (2C), 128.6 (2C), 126.5, 63.0 (1JC–D = 23 Hz), 39.0; HRMS (EI) calcd for [M+˙] C8H8D2O 124.0855, found 124.0857, 106 (7%), 91 (100%).[1-2H1]-2-Phenylethanal (31)46
A similar procedure was employed as that described for the preparation of 20. Thus, reaction of [1,1-2H2]-phenylethanol (30) with IBX (1.70 g, 6.14 mmol) in DMSO (15 mL) gave the aldehyde 31 (450 mg, 91%) after work-up. The crude aldehyde was used without further purification as the aldehyde is unstable at room temperature. Data for unlabelled material: IR (CH2Cl2, cast) 3086, 3062, 3028, 1723, 1603, 1453 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.76 (t, 1H, CHO, J = 2.4 Hz), 7.42–7.21 (m, 5H, Ar), 3.70 (d, 2H, CH2J = 2.4 Hz); HRMS (EI) calcd for [M+˙] C8H8O 120.0575, found 120.0574, 106 (7%), 91 (100%). The labelled aldehyde 31 had similar data to that of the unlabelled material except for the following: 1H NMR (CDCl3, 300 MHz) δ 7.21–7.42 (5H, m, Ar), 3.70 (2H, s, CH2).(1S)-[1-2H1]-2-Phenylethyl tosylate (32)
An oven dried 100 mL round bottom flask was charged with (−)-β-chlorodiisopinocampheylborane (1.55 g, 4.84 mmol) in an inert atmosphere and dissolved in THF (10 mL). The solution was cooled to −78 °C and to this cold solution was added dropwise a solution of [1-2H1]-phenylethanal (31) (4.03 mmol) and stirred with warming to room temperature for 18 h. The solvent was removed in vacuo and α-pinene was removed by high vacuum for 8 h. The residue was dissolved in Et2O and to this stirring solution was added diethanolamine (1.02 mL, 10.65 mmol). The solids were removed by filtration through a pad of Celite® and filtrate was concentrated in vacuo. Purification of the crude alcohol by flash column chromatography (4 : 1 hexanes–EtOAc) gave the alcohol. Data for alcohol: IR (CH2Cl2, cast) 3335, 3085, 3062, 2932, 2158, 1603, 1496 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.29–7.36 (m, 2H, Ar), 7.21–7.27 (m, 3H, Ar), 3.86 (dt, 1H, J = 6.6 Hz, 1.5 Hz, CHDCH2), 2.88 (d, 2H, J = 6.3 Hz, CH2CHD); 13C NMR (CDCl3, 125 MHz) δ 138.5, 129.0 (2C), 128.6 (2C), 126.5, 63.3 (t, 1JC–D = 22 Hz), 39.1; HRMS (EI) calcd for [M+˙] C8H9DO 123.0793, found 123.0795, 106 (3%), 91 (100%), 65 (14%). A stirring solution of the alcohol (107 mg, 0.88 mmol) and tosyl chloride (202 mg, 1.06 mmol) in CH2Cl2 (5 mL) was treated with Et3N (0.16 mL, 1.16 mmol) and DMAP (10 mg 0.08 mmol). The resulting reaction mixture was stirred at room temperature for 18 h, at which time it was diluted with water (10 mL) and extracted with CH2Cl2. The combined organic extracts were washed with water and brine, dried over MgSO4 and concentrated in vacuo. The crude tosylate was purified by flash column chromatography (SiO2, hexane–EtOAc, 10 : 1) to afford the tosylate 32 as an oil (184 mg, 75%). IR (CH2Cl2, cast) 3029, 2924, 2193, 1597, 1362, 1178 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.68 (m, 2H, Ar), 7.19–7.29 (m, 5H, Ar), 7.09 (m, 2H, Ar), 4.18 (m, 1H, CHDCH2), 2.93 (d, 2H, J = 7.1 Hz, CH2CHD), 2.41 (s, 3H, ArCH3); 2H NMR (CH2Cl2, 76.5 MHz) δ 4.23; 13C NMR (CDCl3, 125 MHz) δ 144.6, 136.2, 133.0, 129.8 (2C), 128.8 (2C), 128.6 (2C), 126.8 (2C), 126.9, 70.3, (t, 1JC–D = 23 Hz), 35.3, 21.6; HRMS (EI) calcd for [M+˙] C15H15DO3S 277.0883, found 277.0884, 172 (5%), 155 (15%), 105 (100%), 91 (83%).(1R)-[1-2H1]-2-Phenylethyl tosylate (33)
A similar procedure was employed as that described for the preparation of 32. Treatment of [1-2H]-2-phenylethanal (31) (4.03 mmol) in THF (10 mL) with (+)-β-chlorodiisopinocampheylborane (1.55 g, 4.84 mmol) gave the crude alcohol. Purification using flash column chromatography (SiO2, hexane–EtOAc, 4 : 1) afforded (1R)-[1-2H]-phenylethanol as a colorless oil (200 mg, 40%). IR (CH2Cl2, cast) 3335, 3085, 3062, 2932, 2158, 1603, 1496 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.29–7.36 (m, 2H, Ar), 7.21–7.27 (m, 3H, Ar), 3.86 (dt, 1H, J = 6.6 Hz, 1.5 Hz, CHDCH2), 2.88 (d, 2H, J = 6.3 Hz, CH2CHD); 13C NMR (CDCl3, 125 MHz) δ 138.5, 129.0 (2C), 128.6 (2C), 126.5, 63.3 (t, 1JC–D = 22 Hz), 39.1; HRMS (EI) calcd for [M+˙] C8H9DO 123.0793, found 123.0795. 106 (3%), 91 (100%). Reaction of the alcohol (250 mg, 2.06 mmol) in CH2Cl2 (15 mL) with p-toluenesulfonyl chloride (471 mg, 2.47 mmol), triethylamine (0.38 mL, 2.72 mmol) and DMAP (10 mg, 0.08 mmol) provided the crude tosylate. Purification by flash column chromatography (SiO2, hexane–EtOAc, 10 : 1) afforded 33 as an oil (490 mg, 86%). IR (CH2Cl2, cast) 3030, 2925, 2195, 1653, 1598, 1362, 1176 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.67 (m, 2H, Ar), 7.19–7.29 (m, 5H, Ar), 7.09 (m, 2H, Ar), 4.18 (m, 1H, CHDCH2), 2.93 (d, 2H, J = 7.1 Hz, CH2CHD), 2.41 (s, 3H, ArCH3); 2H NMR (CH2Cl2, 76.5 MHz) δ 4.23; 13C NMR (CDCl3, 125 MHz) δ 144.6, 136.2, 133.0, 129.8 (2C), 128.8 (2C), 128.6 (2C), 127.8 (2C), 126.9, 70.3 (t, 1JC–D = 23 Hz), 35.3, 21.6; HRMS (EI) calcd for [M+˙] C15H15DO3S 277.0883, found 277.0884, 172 (5%), 155 (15%), 105 (100%), 91 (83%).(3R)-[3-2H1]-4-Phenyl-1-butyne (34)
To a stirring solution of (1S)-[1-2H]-2-phenylethyl tosylate (32) (200 mg, 0.721 mmol) in DMSO (5 mL) was added lithium acetylide–ethylene diamine complex (199 mg, 2.16 mmol). The resulting reaction mixture was stirred at room temperature for 2 h and then poured into a solution of cold 1 M HCl to quench the reaction. The insoluble material was removed by filtration through a pad of Celite® and the filtrate was extracted with Et2O. The combined ethereal extracts were washed with water and brine, dried over MgSO4 and evaporation gave the crude alkyne 34. The crude oil was used without any further purification for the preparation of (3R)-[3-2H1]-4-phenyl-2-butanone (36). IR (CH2Cl2, cast) 3304, 3078, 3025, 2976, 2258, 1601, 1494, 1079 cm−1; 1H NMR (CD2Cl2, 400 MHz) δ 7.37–7.40 (m, 2H, Ar), 7.20–7.33 (m, 3H, Ar), 2.83 (d, 2H, J = 7.1 Hz, CHDCH2), 2.48 (m, 1H, CHDCH2), 1.98 (d, 1H, J = 2.6 Hz, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH); 2H NMR (CH2Cl2, 76.5 MHz) δ 2.47; 13C NMR (CD2Cl2, 125 MHz) δ 136.8, 128.5 (2C), 127.8 (2C), 126.3, 83.8, 68.9, 34.8, 20.3 (t, 1JC–D = 20.0 Hz); HRMS (EI) calcd for [M+˙] C10H9D 131.0845, found 131.0846, 105 (43%), 91 (100%).
CH); 2H NMR (CH2Cl2, 76.5 MHz) δ 2.47; 13C NMR (CD2Cl2, 125 MHz) δ 136.8, 128.5 (2C), 127.8 (2C), 126.3, 83.8, 68.9, 34.8, 20.3 (t, 1JC–D = 20.0 Hz); HRMS (EI) calcd for [M+˙] C10H9D 131.0845, found 131.0846, 105 (43%), 91 (100%).
(3S)-[3-2H1]-4-Phenyl-1-butyne (35)
A similar procedure was employed as that described for the preparation of 34. The reaction of (1R)-[1-2H]-2-phenylethyl tosylate (33) (200 mg, 0.721) in DMSO (5 mL) with lithium acetylide–ethylene diamine complex (199 mg, 2.16 mmol) gave the crude (3S)-[3-2H1]-4-phenylbutyne (35). The crude oil was used without any further purification for the preparation of (3S)-[3-2H1]-4-phenyl-2-butanone (37). The enantiomeric alkyne had identical spectral properties to that of (3R)-[3-2H1]-4-phenylbutyne (34).(3R)-[3-2H1]-4-Phenyl-2-butanone (36)
To a stirring solution of (3R)-[3-2H1]-4-phenylbutyne (34) (0.721 mmol) in acetic acid (6.0 mL) and water (1.0 mL) was added mercuric acetate (919 mg, 2.88 mmol). The resulting reaction mixture was stirred at 70 °C for 3 h at which time water (30 mL) was added. The pH of the solution was adjusted to 7 with the addition of sodium acetate. The mixture was cooled to 0 °C in an ice-water bath and sodium borohydride was added carefully. The reaction mixture was then stirred for 30 min and filtered through a pad of Celite,® and the filtrate was extracted with Et2O. The combined ethereal extracts were washed with water and brine, and dried over MgSO4 and concentrated in vacuo. The crude residue was dissolved in CH2Cl2 and treated with PDC (298 mg, 0.79 mmol) and stirred for 3 h at room temperature. The suspension was filtered through a pad of Celite® and the filtrate was concentrated in vacuo to give the crude ketone. Purification by flash chromatography (SiO2, pentanes–Et2O, 10 : 1) to afford the ketone 36 (45 mg, 42%) as a colorless liquid. IR (CH2Cl2, cast) 3027, 2956, 2871, 1717, 1602, 1497, 1162 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.15–7.29 (m, 5H, Ar), 2.86 (d, 2H, J = 7.0 Hz, CH2CHD), 2.74 (1H, m, CH2CHD), 2.12 (3H, s, CH3CO); 2H NMR (CHCl3, 76.5 MHz) δ 2.74; 13C NMR (CDCl3, 125 MHz) δ 207.8, 141.0, 128.5 (2C), 128.3 (2C), 126.1, 45.2, 30.1 (t, 1JC–D = 29 Hz), 29.8; HRMS (EI) calcd for [M+˙] C10H11DO 149.0951, found 149.0944, 134 (30%), 106 (98%), 91 (100%).(3S)-[3-2H1]-4-Phenyl-2-butanone (37)
A similar procedure was employed as that described for the preparation of 36. Treatment of a solution of (3S)-[3-2H1]-4-phenylbutyne (35) (0.721 mmol) in acetic acid (6.0 mL) and water (1.0 mL) with mercuric acetate (919 mg, 2.88 mmol) gave the mercuric salt. Addition of NaBH4 (202 mg, 5.34 mmol) and oxidation by PDC (220 mg, 0.59 mmol) afforded (3S)-[3-2H1]-4-phenyl-2-butanone (37). Purification by flash column chromatography (SiO2, pentanes–Et2O) provided the ketone 37. The enantiomeric ketone had identical spectral properties to that of (3R)-[3-2H1]-4-phenyl-2-butanone (36).(3R)-[3-2H1]-Levulinic acid (38)
A solution of (3R)-[3-2H1]-4-phenyl-2-butanone (36) (106 mg, 0.721 mmol) in CCl4 (4 mL), MeCN (4 mL) and H2O (6 mL) was treated with sodium periodate (1.54 g, 7.21 mmol) and ruthenium trichloride (3 mg, 14 µmol). The resulting reaction mixture was stirred at room temperature for 18 h. The solution was diluted with water and extracted with CH2Cl2. The combined organic extracts were dried with MgSO4 and concentrated in vacuo to give the crude acid 38 (20 mg, 24%). The acid was used for the preparation of (S)-(+)-(methoxycarbonyl)benzyl (3R)-[3-2H1]-levulinate (40) without further purification. For unlabelled material: IR (CH2Cl2, cast) 3111, 1716, 1402, 1369, 1165 cm−1; 1H NMR (CDCl3, 300 MHz) δ 2.73 (m, 2H, CH2CO), 2.61 (m, 2H, CH2COOH), 2.17 (s, 3H, CH3CO); 13C NMR (CDCl3, 100 MHz) δ 206.9, 178.4, 37.7, 29.7, 27.8; HRMS (EI) calcd for [M+˙] C5H8O3 116.0472, found 116.0474, 101 (22%), 73 (47%), 56 (100%). The (3R)-[3-2H1]-levulinic acid (38) showed identical chromatographic and spectral properties except for the following: 2H NMR (C6H6, 61.4 MHz) δ 2.73.(3S)-[3-2H1]-Levulinic acid (39)
A similar procedure was employed as that described for the preparation of 38. Treatment of (3S)-[3-2H1]-4-phenyl-2-butanone (37) (106 mg, 0.721 mmol) in CCl4 (4 mL), MeCN (4 mL) and H2O (6 mL) with sodium periodate (1.54 g, 7.21 mmol) and ruthenium trichloride (3 mg, 0.014 mmol) gave (3S)-[3-2H1]-levulinic acid (39) as a crude mixture. The acid was used without further purification for the preparation of (S)-(+)-(methoxycarbonyl)benzyl (3S)-[3-2H1]-levulinate (41). Spectral data was identical to that reported for 38.(S)-(+)-(Methoxycarbonyl)benzyl (3R)-[3-2H1]-levulinate (40)
To a stirring solution of (3R)-[2-2H1]-levulinic acid (38) (18 mg, 0.154 mmol) in CH2Cl2 (5 mL) was added (S)-(+)-mandelic acid methyl ester (28 mg, 0.17 mmol), DCC (39 mg, 0.19 mmol) and DMAP (2 mg, 0.02 mmol). The resulting reaction mixture was stirred at room temperature for 18 h and filtered through a sintered glass funnel to remove the solid impurities. The crude ester was purified by flash column (SiO2, hexane–EtOAc, 4 : 1) to afford the title compound 40 (16 mg, 41%) as a colorless oil. For unlabelled material: IR (CH2Cl2, cast) 3034, 2954, 1745, 1719, 1587, 1364, 1152 cm−1; 1H NMR (C6D6, 300 MHz) δ 7.42 (m, 2H, Ar), 7.04 (m, 3H, Ar), 6.05 (s, 1H, CHCO2Me), 3.18 (s, 3H, OCH3), 2.50 (m, 2H, J = 7.0 Hz, CH2CO2R), 2.21 (td, 1H, J = 6.3 Hz, 18.2 Hz, CH2CO), 2.01 (td, 1H, J = 6.3 Hz, 18.2 Hz, CH2CO), 2.01 (s, 3H, CH3CO); 13C NMR (CDCl3, 125 MHz) δ 206.1, 172.1, 169.2, 133.7, 129.3, 128.8 (2C), 127.6 (2C), 74.6, 52.6, 37.8, 29.8, 27.8; HRMS (EI) calcd for [M+˙] C14H16O5 264.0998, found 264.0989, 232 (5%), 205 (4%), 166 (7%), 149 (6%), 121 (10%), 99 (100%). The (S)-(+)-(methoxycarbonyl)benzyl (3R)-[3-2H1]-levulinate (40) showed identical chromatographic properties and displayed similar spectral properties except for the following: 2H NMR (C6H6, 76.5 MHz) δ 2.01.(S)-(+)-(Methoxycarbonyl)benzyl (3S)-[3-2H1]-levulinate (41)
A procedure similar to that used for the preparation of (S)-(+)-(methoxycarbonyl)benzyl (3R)-[3-2H1]-levulinate (40) was employed except that (3S)-[2-2H1] levulinic acid (39) was used as the starting material. Spectral data were similar to those of 40 except for 2H NMR (C6H6, 76.5 MHz) δ 2.21.Incorporation studies with rubber transferase
Enzymatically active washed rubber particles from H. brasiliensis47 and P. argentatum6 were purified as previously described. Incorporation assays were performed in duplicate and using a modification of the method by Cornish and Beckhaus.6 A typical reaction took place in 1.0 mL siliconized micro-centrifuge tubes. The total reaction volume was typically 250 µL (100 mM Tris–HCl pH 7.5; 5 mM DTT, 20 µM FPP, 1.25 mM MgSO4, 11 mg washed rubber particles (WRP), and 5 mM of deuterated IPP analog). Reactions were incubated for 4 d at 27 °C for H. brasiliensis or at 16 °C for P. argentatum, and were stopped by the addition of EDTA (0.5 M, pH 8.0). The reaction mixtures were then filtered through 0.22 µm nitrocellulose filters to trap the rubber particles. The rubber (ca. 10 mg) was then washed successively with water (2 × 1 mL), 1 M HCl (3 mL) and EtOH (3 × 4 mL). The newly synthesized rubber was then removed from the filter paper and was either analyzed by 2H NMR or ozonolysis.Ozonolysis of rubber
Rubber isolated from incorporation experiments (typically 10 mg) was dissolved in 10 mL of CH2Cl2 and cooled to −40 °C. Ozone was then bubbled through this solution for 20 min, at which time excess O3 was purged with bubbling O2. The solvent was evaporated in vacuo and to the resulting residue was added formic acid (1 mL) and H2O2 (0.3 mL, 30%). After stirring at room temperature for 4 h, the excess peroxides were decomposed by the addition of solid FeSO4. The solution was diluted with Et2O (5 mL) and extracted with Et2O (2 × 5 mL). The combined ethereal extracts were washed with brine, dried over MgSO4 and concentrated in vacuo to give levulinic acid.Derivatization of enzymatically synthesized levulinic acid
The crude levulinic acid from ozonolysis of rubber was dissolved in CH2Cl2 (10 mL) and to this solution was added (S)-(+)-mandelic acid methyl ester (28 mg, 0.17 mmol), DCC (39 mg, 0.19 mmol) and DMAP (2 mg, 0.02 mmol). The resulting reaction mixture was stirred at room temperature for 18 h and filtered through a sintered glass funnel to remove solid impurities. The crude ester was purified by preparative thin layer chromatography (SiO2, hexane–EtOAc, 4 : 1, Rf = 0.21) to afford the desired ester (2–5 mg).Acknowledgements
The authors would like to acknowledge Dr Deborah Scott, Dr Colleen McMahan and Dr Katrina Cornish (US Department of Agriculture, Agricultural Research Station (USDA-ARS), Albany CA) for the preparation of rubber particles from H. brasiliensis and P. argentatum. This work was supported by the Advanced Food and Biomaterials Network (AFMNet), the Natural Sciences and Engineering Research Council of Canada, the Alberta Heritage Foundation for Medical Research, the Canada Research Chair in Biooorganic Chemistry and Medicinal Chemistry and the USDA.References
- K. Cornish and J. L. Brichta, in Purification of Hypoallergenic Latex from Guayule, ed. J. Janick and A. Whipkey, ASHS Press, 2002, pp. 226–233 Search PubMed.
- H. Mooibroek and K. Cornish, Appl. Microbiol. Biotechnol., 2000, 53, 355–365 CrossRef CAS.
- K. Cornish, Phytochemistry, 2001, 57, 1123–1134 CrossRef CAS.
- K. Cornish, Nat. Prod. Rep., 2001, 18, 182–189 RSC.
- K. Cornish, Eur. J. Biochem., 1993, 218, 267–271 CAS.
- K. Cornish and R. A. Backhaus, Phytochemistry, 1990, 29, 3809–3813 CrossRef CAS.
- B. M. da Costa, J. D. Keasling and K. Cornish, Biomacromolecules, 2005, 6, 279–289 CrossRef CAS.
- J. W. Cornforth, R. H. Cornforth, G. Popjak and L. Yengoyan, J. Biol. Chem., 1966, 241, 3970–3987 CAS.
- J. W. Cornforth, R. H. Cornforth, C. Donninger and G. Popjak, Proc. R. Soc. London, Ser. B, 1966, 163, 492–514 CrossRef CAS.
- B. L. Archer, D. Barnard, E. G. Cockbain, J. W. Cornforth, R. H. Cornforth and G. Popjak, Proc. R. Soc. London, Ser. B, 1966, 163, 519–523 Search PubMed.
- M. Rohmer, Nat. Prod. Rep., 1999, 16, 565–574 RSC.
- G. Flesch and M. Rohmer, Eur. J. Biochem., 1988, 175, 405–411 CAS.
- I. Takahashi and K. Ogura, J. Biochem., 1982, 92, 1527–1537 CAS.
- I. Takahashi, K. Ogura and S. Seto, J. Biol. Chem., 1980, 255, 4539–4543 CAS.
- T. Suga, T. Hirata, T. Aoki and T. Kataoka, J. Am. Chem. Soc., 1986, 108, 2366–2371 CrossRef CAS.
- M. Ito, M. Kobayashi, T. Koyama and K. Ogura, Biochemistry, 1987, 26, 4745–4750 CrossRef CAS.
- M. Kobayashi, M. Ito, T. Koyama and K. Ogura, J. Am. Chem. Soc., 1985, 107, 4588–4589 CrossRef CAS.
- M. Ito, T. Koyama and K. Ogura, Chem. Lett., 1986, 101–104 CAS.
- T. Yoshioka, R.-Y. Zheng, S. Ohta and T. Suga, Phytochemistry, 1990, 29, 3467–3472 CrossRef CAS.
- T. Suga, R.-Y. Zheng, S. Ohta, T. Yoshioka and T. Hirata, Chem. Lett., 1987, 497–500 CAS.
- G. Popjak and J. W. Cornforth, Biochem. J., 1966, 101, 553–568 CAS.
- D. E. Cane, R. Iyengar and M. S. Shiao, J. Am. Chem. Soc., 1981, 103, 914–931 CrossRef CAS.
- D. E. Cane and S. W. Weiner, Can. J. Chem., 1994, 72, 118–127 CAS.
- A. E. Leyes and C. D. Poulter, Org. Lett., 1999, 1, 1067–1070 CrossRef CAS.
- C. Hubschwerlen, Synthesis, 1986, 962–964 CrossRef CAS.
- C. Hubschwerlen, J. L. Specklin and J. Higelin, Org. Synth., 1995, 72, 1–5 CAS.
- A. Meddour, I. Canet, A. Loewenstein, J. M. Pechine and J. Courtieu, J. Am. Chem. Soc., 1994, 116, 9652–9656 CrossRef CAS.
- V. J. Davisson, A. B. Woodside, T. R. Neal, K. E. Stremler, M. Muehlbacher and C. D. Poulter, J. Org. Chem., 1986, 51, 4768–4779 CrossRef CAS.
- M. Frigerio, M. Santagostino and S. Sputore, J. Org. Chem., 1999, 64, 4537–4538 CrossRef CAS.
- M. Frigerio and M. Santagostino, Tetrahedron Lett., 1994, 35, 8019–8022 CrossRef CAS.
- M. M. Midland, S. Greer, A. Tramontano and S. A. Zderic, J. Am. Chem. Soc., 1979, 101, 2352–2355 CrossRef CAS.
- M. M. Ravn, Q. W. Jin and R. M. Coates, Eur. J. Org. Chem., 2000, 1401–1410 CrossRef CAS.
- C. J. Reich, G. R. Sullivan and H. S. Mosher, Tetrahedron Lett., 1973, 1505–1508 CrossRef CAS.
- Model studies of the hydrolysis of the unlabelled nitrile 32 in 12 M DCl indicated that no exchange with solvent hydrogens was observed at the carbon to the nitrile.
- D. Parker, Chem. Rev., 1991, 91, 1441–1457 CrossRef CAS.
- B. J. Rawlings, P. B. Reese, S. E. Ramer and J. C. Vederas, J. Am. Chem. Soc., 1989, 111, 3382–3390 CrossRef CAS.
- K. Arai, B. J. Rawlings, Y. Yoshizawa and J. C. Vederas, J. Am. Chem. Soc., 1989, 111, 3391–3399 CrossRef CAS.
- L. Crombie and A. D. Heavers, J. Chem. Soc., Perkin Trans. 1, 1992, 1929–1937 RSC.
- H. C. Brown, J. Chandrasekharan and P. V. Ramachandran, J. Am. Chem. Soc., 1988, 110, 1539–1546 CrossRef CAS.
- M. B. M. de Azevedo, M. M. Murta and A. E. Greene, J. Org. Chem., 1992, 57, 4567–4569 CrossRef CAS.
- T. Suga, S. Ohta and T. Ohmoto, J. Chem. Soc., Perkin Trans. 1, 1987, 2845–2848 RSC.
- R. J. Crawford, S. B. Lutener and R. D. Cockcroft, Can. J. Chem., 1976, 54, 3364–3376 CAS.
- J. L. Giner and D. Arigoni, Chem. Commun., 2002, 1388–1389 RSC.
- P. R. Andreana, J. S. McLellan, Y. Chen and P. G. Wang, Org. Lett., 2002, 4, 3875–3878 CrossRef CAS.
- K. S. Rangappa, J. Phys. Org. Chem., 2001, 14, 684–690 CrossRef CAS.
- J. A. Kampmeier, S. H. Harris and D. K. Wedegaertner, J. Org. Chem., 1980, 45, 315–318 CrossRef CAS.
- K. Cornish and D. L. Bartlett, Phytochem. Anal., 1997, 8, 130–134 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
