PET modulated fluorescent sensing from the BF2 chelated azadipyrromethene platform†
Michael J.
Hall
,
Lorcan T.
Allen
and
Donal F.
O'Shea
*
Centre for Synthesis and Chemical Biology, Conway Institute, School of Chemistry and Chemical Biology, University College Dublin, Belfield, Dublin 4, Ireland. E-mail: donal.f.oshea@ucd.ie; Tel: +353 (0) 17162425
First published on 19th January 2006
Abstract
A convergent building block synthesis has been applied to new off/on photoinduced electron transfer (PET) modulated fluorescent sensors which are based on a BF2 chelated tetraarylazadipyrromethene platform and operate in the biomedically important red region of the visible spectrum. Incorporation of diethylamine and morpholine receptors facilitates off/on microenvironment polarity and pH sensing. Aqueous formulation and in vitro cellular imaging demonstrates their potential for intracellular sensing.
Introduction
The development of molecular fluorescent sensors for biologically related applications is a very active research area.1 The use of high sensitivity fluorescence detection has become a widely used tool for probing the molecular processes of biological systems in vitro,2 and the application of fluorescence detection to non-invasive in vivo optical imaging is currently emerging.3 The majority of fluorescent molecular sensors have operational light input/output wavelengths in the 300–550 nm wavelength range. In the case of in vivo imaging applications, this spectral region suffers strong interference from endogenous chromophores. In addition, fluorochrome excitation with blue and green light can be damaging to the cellular systems under observation. The most efficient light penetration of biological tissue occurs in the lower energy red and near-infrared (NIR) spectral regions.4 As such, the development of new visible red and NIR off/on fluorescent sensors would be of benefit to future imaging applications. Several research groups have recently reported a number of visible red molecular sensors based on cyanines, squaraines and rhodamines, modified BODIPY dyes and N-phenyl-1,8-naphthalimides.5Herein, we report a new class of fluorescent sensors based upon the BF2 chelated tetraarylazadipyrromethene fluorophore 1 (Fig. 1) which employs a receptor–methylene spacer–fluorophore architecture. We have recently reported that this class of compound displays strong absorption and fluorescence in the 650–750 nm region of the spectrum.6 For example, the parent tetraphenyl analogue of 1 (Ar1–4 = Ph) has an absorption λmax at 650 nm (ε = 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dm−3 mol−1 cm−1) and emission at 672 nm (ϕF = 0.34) in chloroform. These promising photophysical characteristics suggest that these fluorophores would make an excellent platform from which visible red sensors could be constructed.
000 dm−3 mol−1 cm−1) and emission at 672 nm (ϕF = 0.34) in chloroform. These promising photophysical characteristics suggest that these fluorophores would make an excellent platform from which visible red sensors could be constructed.
 | ||
| Fig. 1 BF2 chelated tetraarylazadipyrromethene fluorochromes. | ||
The design of fluorescent sensors most commonly adopts a receptor–spacer–fluorophore architecture with the fluorescence switching properties controlled by a photoinduced electron transfer (PET) mechanism.7 The role of the receptor is to detect the targeted substrate, while the fluorophore quantifies and communicates this information to the observer. The spacer unit covalently links the fluorophore to the receptor whilst keeping the ground state electronic systems of the receptor and the fluorophore disconnected (Fig. 2). This sensing technology can be applied to a diverse set of substrates by the use of substrate specific receptors, allowing analytes such as protons, cations, anions, carbohydrates and peptides to be detected.8
 | ||
| Fig. 2 Schematic of a PET fluorescent sensor design. Rectangle = fluorophore; CH2 = methylene spacer; circle = receptor for substrate; S = substrate. | ||
In order to examine the PET sensing properties of BF2 chelated tetraarylazadipyrromethenes, we synthesised and examined the photophysical properties of the benzylamine substituted analogues 1a and 1b, which would have the potential to act as off/on switching receptors for pH9 and microenvironment polarity (Fig. 3).10
 | ||
| Fig. 3 PET modulated fluorescence molecular sensors. | ||
The synthesis of our target compounds utilised our previously described modular approach, involving the synthesis and condensation of 2,4-diaryl-5-nitrosopyrroles with 2,4-diarylpyrroles.11 Generation of 1a and 1b was accomplished through the condensation of 2-nitroso-3,5-diphenylpyrrole 2 with the receptor substituted pyrroles 3a,b to yield the tetraarylazadipyrromethenes 4a,b. BF2 chelation of 4a,b was achieved by reaction with borontrifluoride diethyletherate with diisopropylethylamine as the base in dichloromethane at room temperature for 16 hours. Purification of the final products was by chromatography on alumina, which provided 1a,b as copper coloured solids in 88 and 91% yields respectively (Scheme 1).
 | ||
| Scheme 1 Synthesis of 1a and 1b. Reagents and conditions: (i) acetic anhydride, acetic acid, 100 °C, 1 h. (ii) BF3·OEt2, DIEA, CH2Cl2, rt, 16 h. | ||
Our expectations for the sensing properties of 1a,b were that little change in the ground state UV–visible spectral properties would be observed on substrate recognition, however, large changes in the fluorescence intensity would act as the sole signalling event. The UV–visible spectra of 1a in four solvents of decreasing dipolarity—DMF, THF, dioxane and cyclohexane—are shown in Fig. 4.12 The wavelengths of the absorption maxima for 1a show little solvent dependency with only a 10 nm hypsochromic shift observed for cyclohexane (647 nm) when compared to DMF (657 nm) (Table 1, Fig. 4). Identical behaviour was recorded for 1b (Table 1, ESI†). Similarly, only a 12 nm difference between the wavelengths of the emission maxima in cyclohexane and DMF was observed for 1a and 1b which mirrored the minor hypsochromic shifts observed for non-polar solvents in the UV–visible spectra (Table 1).
 | ||
| Fig. 4 UV–Visible spectra of 1a in DMF (blue), THF (yellow), 1,4-dioxane (black), cyclohexane (green) at 1 × 10−5 M. | ||
| DMF | THF | Dioxane | Cyclohexane | |
|---|---|---|---|---|
| a Concentration of 1 × 10−5 M. b Concentration of 8 × 10−7 M, excitation 630 nm. c 5 µL of TFA added to a 3 mL sample. | ||||
| 1a | ||||
| Absa | 657 | 653 | 652 | 647 |
| Flub | 680 | 677 | 676 | 668 |
| Abs/H+c | 657 | 655 | 653 | 650 |
| Flu/H+c | 682 | 676 | 677 | 672 |
| 1b | ||||
| Absa | 656 | 654 | 652 | 647 |
| Flub | 681 | 676 | 675 | 669 |
| Abs/H+c | 657 | 656 | 654 | 652 |
| Flu/H+c | 684 | 677 | 678 | 672 |
In contrast to the invariance of the emission wavelength maxima in various solvents, the emission intensity showed a marked response to solvent polarity. The trend observed was that fluorescence intensity increased as a function of decreasing polarity along the series of DMF < THF < dioxane < cyclohexane (Fig. 5). A ninefold enhancement in fluorescence intensity was recorded when the extremes of DMF and cyclohexane were compared. It can be interpreted that in more polar solvents, little fluorescence is observed as the excited state quenching by the PET process is efficient (sensor is off). Whereas in non-polar solvents the PET process is an ineffective competing process for fluorescence emission and the sensor is switched on. The switching effect of microenvironment polarity on the PET mechanism has been observed previously.10b,13
 | ||
| Fig. 5 Fluorescence spectra of 1a in DMF (blue), THF (yellow), 1,4-dioxane (black) and cyclohexane (green). Concentration 8 × 10−7 M, excitation 630 nm, slit widths 5 nm. | ||
The one-dimensional switching of our sensors in response to an acid substrate (i.e. no significant ground state change in response to substrate recognition) is shown in Fig. 6. There is virtually no variance in the peak shape or the wavelength of maximum absorbance in the UV–visible spectra for protonated 1a in any of the four solvents examined (Table 1, ESI). There is a marked response however to the acid analyte in the fluorescence spectra. For example the non-protonated 1a is weakly emissive in DMF solutions but upon protonation with aqueous HCl a strong emission is observed at 682 nm. The increase in fluorescence intensity was greater than eightfold from the off to the on state (Fig. 6).
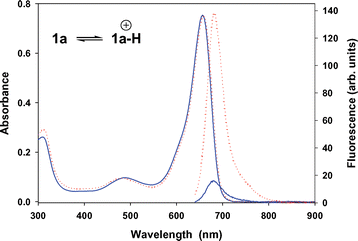 | ||
| Fig. 6 Overlaid UV–visible (1 × 10−5 M) and fluorescence (8 × 10−7 M, excitation 630 nm, slit widths 5 nm) spectra of 1a in DMF (blue) and in DMF–aq. HCl (red). | ||
As would be expected, THF, dioxane and cyclohexane each gave smaller fluorescence intensity enhancements upon the addition of trifluoroacetic acid (ESI). A similar trend for off to on fluorescence enhancement (3-fold) in response to acidic conditions was observed for 1b in DMF (ESI).
In order to obtain aqueous solutions suitable for in vitro imaging, the emulsifier Cremophor EL (CrEL) was used. CrEL is a non-ionic surfactant, frequently used in vivo as a delivery agent for poorly water-soluble drugs such as Taxol.14 The aqueous formulated spectroscopic properties of 1a,b were very similar to those obtained in organic solvents. The fluorescence titration of 1a with aqueous HCl showed a significant increase in fluorescence intensity upon sequential addition of acid aliquots, whereas a more moderate increase was recorded for the morpholine receptor analogue 1b. The titration data predicted apparent pKa values of 6.9 and 4.8, for 1a and 1b respectively, providing a useful window of sensing under physiological conditions (Fig. 7, ESI). It should be noted that pKa values of amines in a micellar microenvironment are often lower than would be anticipated.15
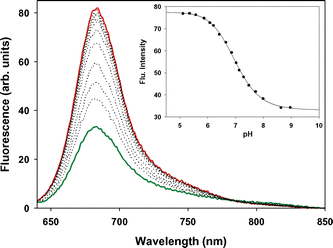 | ||
| Fig. 7 Fluorescence titration of 1a in H2O/CrEL/phosphate buffered saline (INaCl = 150 mmol L−1). Apparent pKa determined to be 6.9 at 25 °C. Red trace pH = 4.9, dark green trace pH = 9. Concentration 8 × 10−7 M, excitation 630 nm, slit widths 5 nm. | ||
To examine the potential of our molecular sensor class for imaging in vitro, an aqueous formulated solution of 1a was incubated with HeLa cells for 1 hour. The cells were washed of surface bound material, slide mounted and imaged using confocal laser scanning microscopy. Cellular localisation of emissive 1a was examined through 16 focal plane sections of 0.48 µm apart through a single cell.16 Analysis of the Z-stack plane views confirmed that emission from 1a was exclusively in the cytoplasm (red) with no fluorescence observed in the nucleus (dark area) (Fig. 8).
 | ||
| Fig. 8 Individual cellular focal plane sections of 1a in a HeLa cell. Cytoplasm – red color; central dark area is the nucleus. Numbers in the left hand corner of each image indicate the cellular position at which the image was recorded. | ||
Each image represents the fluorescence intensity at a specific focal plane of increasing depth through the cell. It can be seen that fluorescence intensity increases as you transect the cell (from 0 to 3 µM), reaches a maximum near the centre of the cell (∼4 to 5 µM) and diminishes as the further edge of the cell is reached. In a cellular context 1a has the potential to come into contact with a variety of different pH ranges and subcellular microenvironment polarities and as such the exact mode of intracellular switching is still under investigation.
Conclusions
We have developed a new fluorophore template for PET based sensing, using the receptor–methylene spacer–fluorophore approach from the boron-difluoro chelated azadipyrromethene platform, with an optical advantage of sensing in the red region of the spectrum. The flexible modular synthesis would readily allow for modified derivatives to be produced to include a range of receptor classes and to fine-tune any desired photophysical parameters. The ability of 1a to localize and be readily detected in vitro gives an indication of the potential of this fluorochrome class as markers for specific cellular events in conjunction with other receptor units and fine-tuning of the switching responses. Future advances in the field of fluorescent molecular sensing and imaging will provide new tools to assist in the understanding of biological processes at the molecular level.Experimental
THF was distilled under N2 over sodium wire and benzophenone. Cyclohexane, dioxane and chloroform were distilled over K2CO3. DMF was distilled under reduced pressure over K2CO3. Solutions for the solvent studies were prepared from a stock solution of 1 (0.005 mmol in 10 mL THF). 1 mL was diluted into 25 mL of either cyclohexane, DMF, dioxane or THF to provide a second stock solution. For UV–visible spectra, 5 mL of the second stock solution was diluted into 10 mL of the relevant solvent to give samples for UV–visible measurements. A 3 mL sample was removed and the UV–visible spectra recorded. 5 µL of trifluoroacetic acid (TFA) was added and the UV–visible spectra were recorded again. For fluorescence spectra 1 mL of the second stock solution was diluted into 25 mL of the relevant solvent to give samples for UV–visible measurements at 8 × 10−7 M. A 3 mL sample was removed and the fluorescence spectra were recorded. 5 µL of TFA was added and the fluorescence spectra were recorded again. Fluorescence measurements were recorded with the following setting; excitation and emission slit widths of 5 nm used, excitation wavelength 630 nm.Synthesis of [3-(4-diethylamino-methylphenyl)-5-phenylpyrrol-2-ylidene]-(3,5-diphenyl-1H-pyrrol-2-yl)-amine BF2 chelate 1a
A stirred solution of [3-(4-diethylamino-methylphenyl)-5-phenylpyrrol-2-ylidene]-(3,5-diphenyl-1H-pyrrol-2-yl)-amine11 (107 mg, 0.2 mmol) in CH2Cl2 (50 mL) was treated with borontrifluoride diethyletherate (350 µL, 2.8 mmol) and diisopropylethylamine (350 µL, 2 mmol). The reaction was stirred at room temperature for 16 h, washed with water (2 × 50 mL), dried over Na2SO4, the solvent was removed under reduced pressure and the resulting solid was purified by chromatography on alumina (CH2Cl2–EtOAc, 4 : 1) to give the product 1a as a copper colored solid (102 mg, 88%); mp 140–142 °C (cyclohexane); λmax(CHCl3)/nm 653 (ε/dm−3 mol−1 cm−1 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000); νmax(KBr disk)/cm−1 3446, 1513; δH(300 MHz; CDCl3; Me4Si) 1.08 (6H, t, J 7.1, N(CH2CH3)2), 2.57 (4H, q, J 7.1, N(CH2CH3)2), 3.63 (2H, s, CH2N), 7.00 (2H, s), 7.43–7.47 (11H, m), 8.01–8.06 (8H, m); δC(75 MHz; CDCl3; Me4Si) 11.8, 46.9, 57.4, 118.8, 119.0, 128.6, 128.6, 129.1, 129.2, 129.4, 129.4, 129.6, 130.8, 130.9, 131.6, 131.7, 132.4, 141.8, 143.9, 144.3, 145.4, 145.8, 159.1, 159.8; m/z (ES) 583.2850 (M + H+. C37H34BF2N4 requires 583.2845).
000); νmax(KBr disk)/cm−1 3446, 1513; δH(300 MHz; CDCl3; Me4Si) 1.08 (6H, t, J 7.1, N(CH2CH3)2), 2.57 (4H, q, J 7.1, N(CH2CH3)2), 3.63 (2H, s, CH2N), 7.00 (2H, s), 7.43–7.47 (11H, m), 8.01–8.06 (8H, m); δC(75 MHz; CDCl3; Me4Si) 11.8, 46.9, 57.4, 118.8, 119.0, 128.6, 128.6, 129.1, 129.2, 129.4, 129.4, 129.6, 130.8, 130.9, 131.6, 131.7, 132.4, 141.8, 143.9, 144.3, 145.4, 145.8, 159.1, 159.8; m/z (ES) 583.2850 (M + H+. C37H34BF2N4 requires 583.2845).
Synthesis of (3,5-diphenyl-1H-pyrrol-2-yl)-[3-(4-morpholin-4-ylmethylphenyl)-5-phenylpyrrol-2-ylidene]-amine BF2 chelate 1b
A stirred solution of (3,5-diphenyl-1H-pyrrol-2-yl)-[3-(4-morpholin-4-ylmethylphenyl)-5-phenylpyrrol-2-ylidene]-amine11 (107 mg, 0.2 mmol) in CH2Cl2 (50 mL) was treated with borontrifluoride diethyletherate (350 µL, 2.8 mmol) and diisopropylethylamine (350 µL, 2 mmol). The reaction was stirred at room temperature for 16 h, washed with water (2 × 50 mL), dried over Na2SO4, the solvent was removed under reduced pressure and the resulting solid was purified by chromatography on silica gel (EtOAc–cyclohexane, 2 : 1) to give the product as a copper colored solid (108 mg, 91%); mp 170–174 °C; λmax(CHCl3)/nm 652 (ε/dm−3 mol−1 cm−1 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000); νmax(KBr disk)/cm−1 3438, 1515; δH(300 MHz; CDCl3; Me4Si) 2.49 (4H, t, J 4.5, N(CH2CH2)2), 3.55 (2H, s, CH2N), 3.74 (4H, t, J 4.5, (CH2CH2)2O), 7.00 (1H, s), 7.01 (1H, s), 7.40–7.48 (11H, m), 8.02–8.06 (8H, m); δC(75 MHz; CDCl3; Me4Si) 53.7, 63.2, 67.0, 118.9, 119.1, 128.6, 129.3, 129.4, 129.5, 129.5, 129.6, 129.6, 130.9, 131.2, 131.6, 132.3, 139.7, 144.0, 144.1, 145.5, 145.7, 159.4, 159.7; m/z (ES) 597.2634 (M + H+, C37H32BF2N4O requires 597.2637).
000); νmax(KBr disk)/cm−1 3438, 1515; δH(300 MHz; CDCl3; Me4Si) 2.49 (4H, t, J 4.5, N(CH2CH2)2), 3.55 (2H, s, CH2N), 3.74 (4H, t, J 4.5, (CH2CH2)2O), 7.00 (1H, s), 7.01 (1H, s), 7.40–7.48 (11H, m), 8.02–8.06 (8H, m); δC(75 MHz; CDCl3; Me4Si) 53.7, 63.2, 67.0, 118.9, 119.1, 128.6, 129.3, 129.4, 129.5, 129.5, 129.6, 129.6, 130.9, 131.2, 131.6, 132.3, 139.7, 144.0, 144.1, 145.5, 145.7, 159.4, 159.7; m/z (ES) 597.2634 (M + H+, C37H32BF2N4O requires 597.2637).
Formulation of 1a for in vitro cellular imaging
Compound 1a (0.005 mmol) was dissolved THF (1 mL), Cremophor EL (0.07 mL) and 1,2-propanediol (0.03 mL) were added and the sample was placed in a sonic bath for 30 min. The THF was removed under reduced pressure and the oily mixture was dissolved in phosphate buffered saline (PBS) solution (10 mL) and filtered through an Acrodisc 25 mm syringe filter (with 0.2 µm HT Tuffryn membrane). The concentration was checked by diluting a portion of the sample (1 mL to 25 mL) with PBS and UV–visible spectral analysis.Confocal laser scanning microscopy
Cells, grown on 8-well chamber slides (Nunc), were incubated in the dark at 37 °C with 1 × 10−5 M 1a for 1 h. Prior to visualisation, excess probe was washed off by rinsing in PBS 4 times and cells were fixed in 3.7% formaldehyde–PBS. Cells were mounted as above and image analysis was performed using an LSM510 META confocal laser scanning microscope (Zeiss) equipped with a 40X numerical aperture 1.0 objective, with a pinhole of 100 µm in diameter being used to capture each image at a resolution of 512 × 512 pixels. 1a was excited by a 543 nm helium neon laser.Acknowledgements
This research was supported by the Program for Research in Third-Level Institutions administered by the HEA. Thanks to Dr D. Rai of the CSCB Mass Spectrometry Centre for analyses.References
- (a) B. Valeur, Molecular Fluorescence Principles and Applications, Wiley-VCH, Weinhreim, 2002 Search PubMed; (b) J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Kluwer, New York, 2nd edn., 1999 Search PubMed.
- P. Mitchell, Nat. Biotechnol., 2001, 19, 1013 CrossRef CAS.
- V. Ntziachristos, J. Ripoll, L. V. Wang and R. Weissleder, Nat. Biotechnol., 2005, 23, 313 CrossRef CAS.
- R. Weissleder, Nat. Biotechnol., 2001, 19, 316 CrossRef CAS.
- For examples see: (a) X. Peng, F. Song, E. Lu, Y. Wang, W. Zhou, J. Fan and Y. Gao, J. Am. Chem. Soc., 2005, 127, 4170 CrossRef CAS; (b) E. Sasaki, H. Kojima, H. Nishimatsu, Y. Urano, K. Kikuchi, Y. Hirata and T. Nagano, J. Am. Chem. Soc., 2005, 127, 3684 CrossRef CAS; (c) Z. Shen, H. Röhr, K. Rurack, H. Uno, M. Spieles, B. Schulz, G. Reck and N. Ono, Chem.–Eur. J., 2004, 10, 4853 CrossRef CAS; (d) W. Pham, R. Weissleder and C.-H. Tung, Angew. Chem., Int. Ed., 2002, 41, 3659 CrossRef CAS; (e) A. L. Ellis, J. C. Mason, H.-W. Lee, L. Strekowski, G. Patonay, H. Choi and J. J. Yang, Talanta, 2002, 56, 1099 CrossRef CAS; (f) K. Rurack, M. Kollmannsberger and J. Daub, Angew. Chem., Int. Ed., 2001, 40, 385 CrossRef CAS; (g) K. Rurack, M. Kollmannsberger and J. Daub, New J. Chem., 2001, 25, 289 RSC; (h) A. Burghart, L. H. Thoresen, J. Chen, K. Burgess, F. Bergström and L. B.-Å. Johansson, Chem. Commun., 2000, 2203 RSC; (i) B. Ozmen and E. U. Akkaya, Tetrahedron Lett., 2000, 41, 9185 CrossRef CAS; (j) K. Rurack, U. Resch-Genger, J. L. Bricks and M. Spieles, Chem. Commun., 2000, 2103 RSC; (k) O. O. Abugo, R. Nair and J. R. Lakowicz, Anal. Biochem., 2000, 279, 142 CrossRef CAS; (l) Y. G. Isgor and E. U. Akkaya, Tetrahedron Lett., 1997, 38, 7417 CrossRef CAS; (m) A. Minta, J. P. Y. Kao and R. Y. Tsien, J. Biol. Chem., 1989, 264, 8171 CAS.
- (a) J. Killoran, L. Allen, J. F. Gallagher, W. M. Gallagher and D. F. O'Shea, Chem. Commun., 2002, 1862 RSC; (b) A. Gorman, J. Killoran, C. O'Shea, T. Kenna, W. M. Gallagher and D. F. O'Shea, J. Am. Chem. Soc., 2004, 126, 10619 CrossRef CAS; (c) W. M. Gallagher, L. T. Allen, C. O'Shea, T. Kenna, M. Hall, J. Killoran and D. F. O'Shea, Br. J. Cancer, 2005, 92, 1702 CrossRef CAS; (d) S. O. McDonnell, M. J. Hall, L. T. Allen, A. Byrne, W. M. Gallagher and D. F. O'Shea, J. Am. Chem. Soc., 2005, 127, 16360 CrossRef.
- (a) A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef; (b) L. Fabbrizzi and A. Poggi, Chem. Soc. Rev., 1995, 24, 197 RSC; (c) B. Valeur, in Topics in Fluorescence Spectroscopy, Probe Design and Chemical Sensing, ed. J. R. Lakowicz, Plenum, New York, 1994, 4, 21 Search PubMed.
- J. H. Hartley, T. D. James and C. J. Ward, J. Chem. Soc., Perkin Trans. 1, 2000, 3155 RSC.
- For examples of other PET modulated pH sensors see: (a) E. Arunkumar and A. Ajayaghosh, Chem. Commun., 2005, 599 RSC; (b) T. Gunnlaugsson, J. P. Leonard, K. Sénéchal and A. J. Harte, J. Am. Chem. Soc., 2003, 125, 12062 CrossRef CAS; (c) L. M. Daffy, A. P. de Silva, H. Q. N. Gunaratne, C. Huber, P. L. M. Lynch, T. Werner and O. S. Wolfbeis, Chem.–Eur. J., 1998, 4, 1810 CrossRef CAS; (d) L. Fabbrizzi, F. Gatti, P. Pallavicini and L. Parodi, New J. Chem., 1998, 1403 RSC; (e) R. A. Bissell, E. Calle, A. P. de Silva, S. A. de Silva, H. Q. N. Gunaratne, J.-L. Habib-Jiwan, S. L. A. Peiris, R. A. D. D. Rupasinghe, T. K. S. D. Samarasinghe, K. R. A. S. Sandanayake and J.-P. Soumillion, J. Chem. Soc., Perkin Trans. 2, 1992, 1559 RSC; (f) A. P. de Silva and R. A. D. D. Rupasinghe, J. Chem. Soc., Chem. Commun., 1985, 1669 RSC.
- For examples of other PET modulated polarity sensors see: (a) R. A. Bissell, A. J. Bryan, A. P. de Silva and C. P. McCoy, J. Chem. Soc., Chem. Commun., 1994, 405 RSC; (b) R. A. Bissell, A. P. de Silva, W. T. M. L. Fernando, S. T. Patumathavithana and T. K. S. D. Samarasinghe, Tetrahedron Lett., 1991, 32, 425 CrossRef CAS; (c) M. S. Fernandez and P. Fromherz, J. Phys. Chem., 1977, 81, 1755 CrossRef.
- M. J. Hall, S. O. McDonnell, J. Killoran and D. F. O'Shea, J. Org. Chem., 2005, 70, 5571 CrossRef CAS.
- M. J. Kamlet, J.-L. M. Abboud, M. H. Abraham and R. W. Taft, J. Org. Chem., 1983, 48, 2877 CrossRef CAS.
- (a) C. J. Fahrni, L. Yang and D. G. VanDerveer, J. Am. Chem. Soc., 2003, 125, 3799 CrossRef CAS; (b) B. Bag and P. K. Bharadwaj, J. Phys. Chem. B, 2005, 109, 4377 CrossRef CAS.
- H. Gelderblom, J. Verweij, K. Nooter and A. Sparreboom, Eur. J. Cancer, 2001, 37, 1590 CrossRef CAS.
- A. Roque, F. Pina, S. Alves, R. Ballardini, M. Maestri and V. Balzani, J. Mater. Chem., 1999, 9, 2265 RSC.
- D. M. Shotton, J. Cell Sci., 1989, 94, 175 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra for 1a and 1b. UV–Visible and fluorescence solvent studies for 1a and 1b. See DOI: 10.1039/b514788c |
| This journal is © The Royal Society of Chemistry 2006 |
