Microbubbles with exceptionally long life—synergy between shell and internal phase components
Frédéric
Gerber
ab,
Marie
Pierre Krafft
*a,
Gilles
Waton
a and
Thierry F.
Vandamme
b
aSystèmes Organisés Fluorés à Finalités Thérapeutiques (SOFFT), Institut Charles Sadron (CNRS UPR 22), 6 rue Boussingault, 67083, Strasbourg, Cedex, France. E-mail: krafft@ics.u-strasbg.fr; Fax: +33 (0)3 41 40 99; Tel: +33 (0)3 41 40 60
bLaboratoire de Chimie Bioorganique (UMR 7514), Université Louis Pasteur, 74 Route du Rhin, 67401, Illkirch, France
First published on 10th March 2006
Abstract
Highly effective stabilization of microbubbles has been obtained by using a fluorocarbon as part of their filling gas and a fluorinated phospholipid, instead of a standard phospholipid, as a shell component. An unexpected strong synergistic effect between the fluorocarbon gas and the fluorinated phospholipid has been discovered.
Introduction
We report an unexpected, strong synergy between a fluorinated lipid constituting the shell component of a microbubble and an internal fluorocarbon gas component. This synergy led to microbubbles that have a half-life in an ultrasound field that can reach 70 min. This is one order of magnitude longer than for microbubbles formulated with the same fluorocarbon in the internal gaseous phase, but without the fluorinated amphiphile in their shell.Ultrasound (US) imaging is the most commonly used diagnostic imaging modality. Contrast agents for US imaging consist of compressible gas microbubbles that scatter US several orders of magnitude more effectively than any other materials.1–5 In addition to enhancing the intensity of backscatter signals, microbubbles interact with sound waves. The response of microbubbles to US is non-linear, generating useful harmonic and sub-harmonic sound waves. Microbubbles can also be destroyed by an US pulse. All these phenomena are being exploited in various new imaging modalities.1–5 Perfluorocarbon-stabilized microbubbles are now also being investigated as oxygen delivery systems (blood substitutes).6,7
However, air bubbles dissolve rapidly in the blood under the combined action of Laplace pressure, arterial pressure, and oxygen metabolism. Microbubbles can be stabilized against both dissolution and coalescence by their shell. An elastic solid polymeric shell can oppose the effect of surface tension and can provide resistance to gas permeation. Diverse hard shells (made of gelatin, alginate, poly(ter-butyloxycarbonylmethyl)glutamate,8 the biodegradable block copolymer poly(D,L-lactide-co-glycolide),9 polyelectrolyte multilayers,10etc.) have been investigated. Phospholipids are also being used as shell components.1–5,11,12 It has been demonstrated that the resistance of the phospholipid monolayer shell to gas permeation was a significant contributor to the stability of air-filled microbubbles.11,12 This contribution is particularly effective when the phospholipid is in the condensed state and increases with the phospholipid’s chain length. However, the half-lives of these bubbles in partially degassed water do not exceed a few minutes.11
Alternately, micron-size bubbles can be stabilized using a perfluorocarbon (PFC) gas. PFCs, when used as part of the filling gas, retard bubble dissolution very effectively, due to very low water solubility.1–5,13 The lifetime of identically formulated, phospholipid-coated microbubbles was shown to increase with increasing molecular weight of the PFC and could be expanded by a factor of 20.13 Due to high biological inertness, high oxygen solubility and extremely low solubility in water, PFCs are being investigated for a range of biomedical uses, including intravascular oxygen transport,6 diagnosis and drug delivery.1,14–17
We have now found that use of the perfluoroalkylated glycerophosphatidylcholine F-GPC as a shell component remarkably prolongs the half-life in an US field of microbubbles filled with perfluorohexane (PFH)-saturated nitrogen. This half-life can reach ∼70 min.
The bilayer gel-to-fluid phase transition temperature, Tc, of F-GPC (Scheme 1) is lower than 5 °C.18 Owing to the specific surface properties of fluorinated chains,14,15F-GPC is expected to decrease surface tension, hence Laplace pressure, more effectively than standard phospholipids. Moreover, fluorinated phospholipids have been shown to form highly stable liposomes that have low shell permeability.14–18 Also noteworthy is the low acute toxicity of F-GPCs, with intravascular LD50 values in the gram range in mice.18 Dimyristoyl phosphatidylcholine (DMPC, Tc ∼ 23 °C) was chosen as the control phospholipid because it was essential, in order to determine the effect of the fluorinated chains, that both test and control materials were in the fluid state.
 | ||
| Scheme 1 Molecular formula of the fluorinated phospholipid used to stabilize the microbubbles. | ||
Results and discussion
The microbubbles investigated here contained either pure N2, or PFH-saturated N2 as filling gases. They were obtained by sonication of an aqueous dispersion of F-GPC, or of DMPC (control), the volume above the dispersion being filled either with PFH-saturated N2 or with pure N2. The time of sonication was adjusted in order to obtain similarly sized microbubbles. The average diameter of the microbubbles, as well as the intensity of the US transmitted through a microbubble-containing cell (Fig. 1), were measured with time. | ||
| Fig. 1 Experimental setting for investigating microbubbles submitted to ultrasound. The emitting transducer is excited with five sinusoids (1; 2.2 MHz, 20 V peak-to-peak) with a repetitive frequency of 100 Hz. The ultrasound signal (2) goes through a glass cell (140 mL) containing the test material, and is received by a second transducer that converts it into an electrical signal (3). | ||
Fig. 2 shows the transmitted US intensity, which reflects the lifetime of the microbubbles under US irradiation, and hence their stability, as a function of time for similarly sized (∼14 μm) PFH-stabilized microbubbles encapsulated in F-GPC or DMPC shells. The half-life of bubbles with F-GPC shells was ∼70 min, as compared to ∼5 min for the reference bubbles with a DMPC shell.
 | ||
| Fig. 2 Variation of the transmitted ultrasound intensity, (I0 is the ultrasound intensity in the absence of bubbles) at 25 °C, as a function of time, for PFH-containing microbubbles stabilized with (a) DMPC and (b) F-GPC. The mean diameter of all the microbubbles is ∼14 μm. The half-lives are 5 and 70 min for the microbubbles with shells made of DMPC and F-GPC, respectively. | ||
Contrary to the regular monotonic curve found with DMPC shells, the curve for F-GPC shells presents two regimes, with an initial slow increase of the US signal intensity from ∼0 to only 20% during the first 60 min, followed by a much faster increase of signal intensity. The second regime, although comparable to the DMPC profile in shape, occurred at a slower rate. This suggests different mechanisms of diffusion of the internal N2/PFH gas for the DMPC- and F-GPC-coated microbubbles.
The half-life of bubbles filled with only N2 was also measured. Their lifetime was found to be less than 100 s, independently of whether the shell was made of F-GPC or of DMPC. Altogether, microbubbles that have both F-GPC in their shell and PFH in their filling gas were found to last about 1 order of magnitude longer than PFH-containing microbubbles of the same size having a DMPC shell. The new bubbles have a 2 orders of magnitude longer half-life than those filled with only N2, independently of whether the latter had a F-GPC or a DPPC shell. These data establish the existence of a hitherto unknown and unpredicted synergistic effect between filling gas and shell component. It is noteworthy that neither F-GPC nor DMPC are in the condensed state. Therefore there is no contribution of a condensed state shell stabilization.
Particle size measurements over time also showed dramatic differences in behavior between F-GPC- and DMPC-coated PFH-stabilized microbubbles (Fig. 3). The mean diameter of the DMPC microbubbles decreased from ∼14 μm to ∼4 μm in only about 10 min (Fig. 3b), whilst it took 60 min for the mean diameter of the F-GPC microbubbles to decrease from ∼14 μm to ∼3 μm (Fig. 3a).19
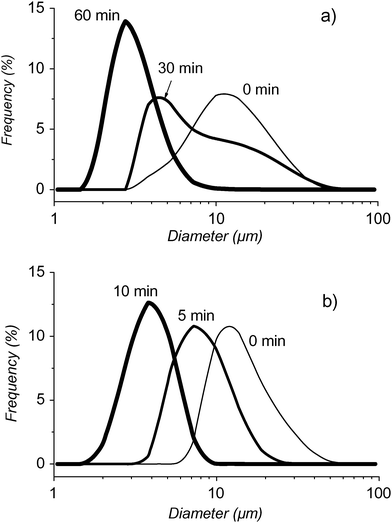 | ||
| Fig. 3 Size distributions of microbubbles filled with N2 saturated with PFH and (a) having a shell made of F-GPC, measured immediately after dilution of the initial foam, then after 30 and 60 min, or (b) having a shell made of DMPC at 0, 5 and 10 min. Temperature was 25 °C. | ||
These size measurements are supported by the US transmission experiments. For DMPC microbubbles, about 80% of the US signal intensity is recovered within 10 min and corresponds to a decrease in bubble size from 14 to 4 μm. By contrast, the F-GPC bubbles remain in this size range (14 to 3 μm) for 60 min. After this time-period, still only 25% of the US signal is transmitted, due to the possibility that long-living small F-GPC-bubbles absorb US very effectively. These results establish that the nature of the shell strongly influences the diffusion rate of the internal gas phase.
The synergistic effect between filling gas and fluorinated amphiphile is further supported by the size analysis of the microbubbles, which shows a significant change in average size and size distribution (Fig. 4). After 30 s of sonication, the diameter and distribution of DMPC-based microbubbles is not significantly different, whether they are filled with pure N2 or with PFH-saturated N2.
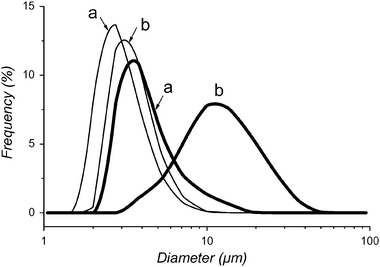 | ||
| Fig. 4 Initial size distributions of microbubbles prepared from fresh foam and having a shell made of DMPC (thin lines) or of F-GPC (thick lines) filled with (a) N2 and (b) N2 saturated with PFH. Temperature was 25 °C. | ||
When prepared from fresh foam, this diameter is typically around 3 μm.20 On the other hand, the diameter of the microbubbles that contain F-GPC in their shells depends strongly on the nature of the filling gas. In the absence of PFH, the mean microbubble diameter is ∼3 μm, comparable to that observed with DMPC, whilst it is ∼14 μm in the presence of PFH, independently of sonication time.
The above synergistic stabilization effect, which allows long-lived microbubbles to be produced, should be of value for US diagnostic, in particular for the detection of deep buried tumors. They may also be useful for intravascular oxygen and drug delivery, where the use of microbubbles is promising.1,6,7,21
Experimental
Perfluorohexane (C6F14, purity >99%), D,L-α-1,2-dimyristoyl-sn-3-glycero-phosphatidylcholine (DMPC, purity >99%) and Pluronic F-68 (MW ∼8300, poly(propylene glycol)/poly(ethylene glycol) = 1 ∶ 4, purity >99%) were purchased from Sigma. The perfluoroalkylated phosphatidylcholine F-GPC was synthesized according to ref. 22. Water was purified using a Millipore system (pH = 5.5; surface tension: 72.1 mN m−1 at 20 °C, resistivity: 18 MΩ cm).The microbubble shell components, F-GPC or DMPC (24 mM), were first allowed to fully rehydrate at 25 °C under agitation during 2–4 h in a phosphate buffered saline (PBS) solution consisting of 121.5 mM NaCl, 25.2 mM Na2HPO4 and 4.8 mM NaH2PO4 (all components from Fluka). The pH of the PBS solution was 7.4. The PBS solution also contained Pluronic F-68 (2.4 mM) as a wetting agent. The dispersions were then sonicated (3 mm probe, 600 W, 20 kHz) by positioning the tip of the probe on top of the solution, the volume above the dispersion being filled either with PFH-saturated N2 or with N2. The time of sonication was adjusted in order to obtain similarly sized microbubbles (30 s for F-GPC and 5 s for DMPC microbubbles filled with PFH-saturated N2). The average diameter of the microbubbles was measured by static light scattering, using Fraunhofer theory, after dilution of the resulting foam in PBS (5 μL in 10 mL of PBS, 0.05% v/v) and with time.
Measurements of the intensity of the US transmitted through a microbubble suspension was achieved using the experimental setup illustrated in Fig. 1. The volume of microbubbles injected in the cell was 50 μL (0.036% v/v), which corresponds to about 2 × 107 bubbles. The microbubbles were maintained under mechanical agitation during the measurement. Ultrasound does not change the bubble dissolution rate at the weak pressure (0.1 atm) that was used. The transmitted US intensity is proportional to the number of microbubbles present between the PZT transducers. Its measurement with time provides directly the lifetime of the microbubbles under US irradiation. All experiments have been performed in triplicate at 25 °C.
Acknowledgements
The authors gratefully acknowledge the Centre National de la Recherche Scientifique (CNRS) for funding.References
- E. G. Schutt, D. H. Klein, R. M. Mattrey and J. G. Riess, Angew. Chem., Int. Ed., 2003, 42, 3218 CrossRef CAS.
- J. G. Riess, Curr. Opin. Colloid Interface Sci., 2003, 8, 259 Search PubMed.
- J. R. Lindner, Nat. Rev. Drug Discovery, 2004, 3, 527 CrossRef CAS.
- Ultrasound Contrast Agents, ed. B. B. Goldberg, J. S. Raichlen and F. Forsberg, Martin Dunitz, London, 2001 Search PubMed.
- E. C. Unger, T. Porter, W. Culp, R. Labell, T. Matsunaga and R. Zutshi, Adv. Drug Delivery Rev., 2004, 56, 1291 CrossRef CAS.
- J. G. Riess, Chem. Rev., 2001, 101, 2797 CrossRef CAS.
- C. E. Lundgren, G. W. Bergoe and I. Tyssebotn, Undersea Hyperb. Med., 2004, 31, 105 Search PubMed.
- M. Schneider, P. Bussat, M. B. Barrau, F. Bodino, C. Gotti, E. Hybl, M. L. Pelaprat and F. Yan, Invest. Radiol., 1991, 26, S190.
- F. Forsberg, J. D. Lathia, D. A. Merton, J.-B. Liu, N. T. Le, B. B. Goldberg and M. A. Wheatley, Ultrasound Med. Biol., 2004, 30, 1281 Search PubMed.
- D. G. Shchukin, K. Köhler, H. Möhwald and G. B. Sukhorukov, Angew. Chem., Int. Ed., 2005, 44, 3310 CrossRef CAS.
- M. A. Borden and M. L. Longo, Langmuir, 2002, 18, 9225 CrossRef CAS.
- G. Pu, M. L. Longo and M. A. Borden, J. Am. Chem. Soc., 2005, 167, 6524 CrossRef.
- A. Kabalnov, J. Bradley, S. Flaim, D. Klein, T. Pelura, B. Peters, S. Otto, J. Reynolds, E. Schutt and J. Weers, Ultrasound. Med. Biol., 1998, 24, 751 CrossRef CAS.
- J. G. Riess, in Handbook of Fluorous Chemistry, ed. J. A. Gladysz, I. Horváth and D. P. Curran, Wiley-VCH, Weinheim, 2004, p. 521 Search PubMed.
- J. G. Riess, Tetrahedron, 2002, 58, 4113 CrossRef CAS.
- J. G. Riess, J. Drug Target., 1994, 21, 87 Search PubMed.
- M. P. Krafft, Adv. Drug Delivery Rev., 2001, 47, 209 CrossRef CAS.
- J. G. Riess, F. Frézard, J. Greiner, M. P. Krafft, C. Santaella, P. Vierling and L. Zarif, in Liposomes – Non-Medical Applications, ed. Y. Barenholz and D. Lasic, CRC Press, Boca Raton, FL, 1996, vol. VI, ch. 8, p. 97 Search PubMed.
- It was not possible to measure bubble sizes smaller than ∼3 μm precisely, likely because the rate of deflation of the microbubbles becomes too fast to allow size measurement by light diffusion techniques.
- In order to obtain 14 μm DMPC microbubbles, the sonication time had to be reduced from 30 s to 5 s.
- A. M. Morawski, G. A. Lanza and S. A. Wickline, Curr. Opin. Biotechnol., 2005, 16, 89 CrossRef CAS.
- C. Santaella, P. Vierling and J. G. Riess, New J. Chem., 1991, 15, 685 Search PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2006 |
