Neoteric optical media for refractive index determination of gems and minerals
Maggel
Deetlefs
a,
Kenneth R.
Seddon
a and
Michael
Shara
b
aThe QUILL Centre, David Keir Building, Stranmillis Road, Belfast, Northern Ireland, UK BT9 5AG. E-mail: quill@qub.ac.uk
bAmerican Museum of Natural History, Division of Physical Sciences, Central Park West and 79th St., New York, NY 10024, USA. E-mail: mshara@amnh.org
First published on 31st January 2006
Abstract
Refractive index determination of minerals and gems often requires their immersion in fluids with the same refractive index. However, these natural materials frequently have refractive indices above the ranges of common organic solvents. Most available high refractive index immersion materials are solid at room temperature, toxic, noxious, corrosive, carcinogenic, or any combination thereof. Since the physical properties of ionic liquids can be tuned by varying the cation and/or anion, we have developed immersion fluids for mineralogical studies which are relatively benign. We report here the syntheses of a range of ionic liquids (many novel) based on the 1-alkyl-3-methylimidazolium cation, which all have refractive indices greater than 1.4, and can be used as immersion fluids for optical mineralogy studies. We further show that for a series of ionic liquids with the same anion, the refractive indices can be adjusted by systematic changes in the cation.
Introduction
The IUPAC definition of refractive index (RI) is ‘the ratio of the speed of light in vacuum to that in a given medium’.1 The refractive index of a substance describes its ability to refract light as it moves from one medium to another and, therefore, the higher the refractive index of a compound, the more the light is refracted.2 In general, higher density materials have greater concentrations of loosely bound electrons and thus larger refractive indices. For most practical purposes, a high refractive index is considered to be a value greater than 1.6 (i.e. above the range of most common organic materials).Amongst gems, diamonds (which have a refractive index of 2.42) are of great interest to earth scientists, since they represent the deepest available samples3 of the earth’s interior, forming by crystallisation in the mantle at depths greater than 150 km. Since diamonds (as well as other minerals) form by crystallisation, they often contain inclusions of foreign material, such as gases, liquids or even other minerals that become trapped within the host mineral during crystallisation (Fig. 1). Often regarded as flaws by the jewellery industry, inclusions provide earth scientists with valuable clues regarding the pressure, temperature, and chemical conditions in which minerals grew, thus serving as hallmarks of the processes which gave rise to them.
 | ||
| Fig. 1 A purple pyrope garnet inclusion hosted in a diamond octahedron.3 | ||
In order to non-destructively locate inclusions in unpolished diamonds and other minerals by optical means, it is desirable to immerse these minerals in a fluid with the same refractive index. Unfortunately, however, many available high refractive index immersion compounds, such as AsI3 (RI = 2.2) or SnI4 (RI = 2.1),4 are solid at room temperature, poisonous, unstable, and extremely unpleasant. Diiodomethane saturated with sulfur5 is another commercially available high refractive index (1.78) room temperature liquid, but this is also a harmful material.
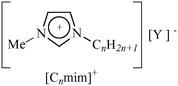
In an attempt to assist in the optical examination of minerals, we were interested in developing ionic liquids (ILs) based on the most common cations viz. 1-alkyl-3-methylimidazolium [Cnmim]+, (Table 1).
|
|
|
|---|---|
| n | Y |
| a Previously unreported ionic liquid. b Tetrafluoroborate. c Bis{(trifluoromethyl)sulfonyl}amide. d Trifluoromethanesulfonate. e Iodide. f Bromide. | |
| 2 | [IBr2], [BrI2], [ClI2], [I7],a [I9]a |
| 4 | [IBr2], [BrI2], [ClI2],a [Br3], [I3], [I5],a [I7],a [I9],a[BF4],b [NTf2],c [OTf]d |
| 6 | I,e [IBr2],a [BrI2],a [I3]a |
| 8 | Brf |
Room temperature ionic liquids are solvents composed entirely of ions with melting points below ca. 100 °C. The burgeoning interest in these non-volatile liquids has resulted in publication rates of (currently) 1000 papers per year, and many research books6,7 and reviews.8,9 Ionic liquids have also been described as ‘designer solvents’,10 since careful selection of the cation, anion, or both, allows the preparation of ionic liquids with specific physical properties. At present, however, physical property data for ionic liquids are still fairly limited, placing restrictions on effective interrogation of existing information to allow predictive ionic liquid preparation. Using the tuneability of ionic liquids, we report here a set of fluids which have been designed to have refractive indices >1.4, have low environmental impact and good shelf life. As high atomic number is associated with high refractive index, ionic liquids containing periodide anions became the cynosure of the investigation. The investigated ionic liquids thus contain various polyhalide anions, and exhibit higher refractive indices than common organic solvents. Although a number of ionic liquids consisting of 1-alkyl-3-methylimidazolium cations and triiodide anions,11 as well as other mixed trihalide anions,12 have previously been described, many of the ionic liquids prepared in this study (see Table 1) are, to the best of our knowledge, new materials.
Experimental
Materials
The ionic liquids, [C4mim][BF4],13 [C4mim][CF3SO3],14 [C4mim][NTf2],15 [C4mim]Cl, [C4mim]Br, and [C4mim]I, [C6mim]Br, and [C6mim]I, as well as [C8mim]I,16 were prepared following published procedures. Elemental bromine (99.99+%), iodine (99.8%) and iodine(I) chloride (>99.5%), all ex. Aldrich, were used as received.Methods and equipment
All syntheses were carried out under a dinitrogen atmosphere using standard glove-box, Schlenk-line, and vacuum-line techniques. Proton nuclear magnetic resonance (1H-NMR) spectra were recorded (300 MHz, δ reported relative to TMS) on a Bruker Advance DPX 300 NMR spectrometer. Elemental analyses were carried out by the Analytical Services and Environmental Projects Unit (ASEP) at the Queen’s University of Belfast, using a Perkin Elmer 2400 CHN Elemental Analyser. A Perkin Elmer Pyris-1 power compensation differential scanning calorimeter was used to determine the melting points of the room temperature ionic liquids and a power compensation Perkin Elmer Pyris-7 DSC was used to determine the melting points of solid samples. The scan rates used were 5 or 10 °C min−1. Electrospray ionisation (ESI) mass spectral data were recorded using a Thermo-Finnagin Surveyor MSQ mass spectrometer at the QUESTOR Centre, the Queen’s University of Belfast (QUB). Liquid Secondary Ion Mass Spectrometry mass spectra (LSIMS) were recorded at the EPSRC National Mass Spectrometry Service Centre, University of Wales, Swansea.17 A MARS-S multimode microwave oven18 was used for the preparation of the 1-alkyl-3-methylimidazolium halide salts. Chloride and water contents were respectively determined using ion chromatography19 and Karl Fischer titration.20,21Ionic liquid syntheses
Optical microscopy methods
 | (1) |
Interferometry
 | ||
| Fig. 2 Schematic representation of a Fabry–Perot interferometer. | ||
We employed a Fabry–Perot interferometric refractive index sensor manufactured by FISO Technologies of Quebec City, Canada to measure ionic liquid refractive indices. The sensor consists of two miniature fibre-optic refractive index transducers, with 0.8 mm outer diameter and 10 mm length Pyrex tubes at each tip. One transducer was optimised for refractive index measurements in the 1.3 to 1.8 range, and a second was optimised for refractive indices between 1.8 and 2.5. A model FTI-10 signal conditioner was coupled to the transducers. The signal conditioner fed an 800 nm light source through each fibre to the transducer tip. It is this near-infrared light that was multiply reflected between the partially silvered glass plates of Fig. 2 to produce the interferogram, which the sensor and detector electronics interpreted.
The sensor of an interferometer consists of two closely spaced, partially-silvered surfaces with spacing, d. The FISO interferometer we used has d = 10 microns, and operates at 800 nm wavelength. The combination of a very small light-absorbing pathlength and infrared wavelength allowed refractive index measurements of the intensely coloured ionic liquids.
When light reaches the silvered surfaces, part of it is transmitted each time the light reaches the second surface, resulting in multiple offset beams. The large number of interfering rays produces an interferogram with extremely high resolution. When the sensor of the interferometer is immersed in a liquid, the value of d becomes d/RI. For Bragg’s law,25 2dcos(α) = mλ (α = angle of incidence of light on the cavity, m = integer at successive interference maxima, and λ = wavelength of light used), to continue to hold true, the value of m must change. The new value of m is determined by the sensor and detector electronics. This is achieved by measuring the widths of the fringes and cross-correlating the fringe pattern with itself. In this way, the refractive index of any liquid, regardless of its appearance, can be determined.
Mineral images
The images of the four investigated minerals, viz. colourless quartz (RI = 1.54), orange beryl (RI = 1.59), brown corundum (RI = 1.76), and cubic zirconia (RI = 2.16), were obtained from a Nikon digital camera fitted with a 150 mm macro lens. The four minerals were placed in a microbeaker of 19 mm diameter (ex. Cargille). This arrangement was housed in a light box illuminated by fluorescent lights. The fluids in which the minerals were immersed were air (RI = 1.00), water (RI = 1.33), [C8mim]Br (RI = 1.49), and [C4mim][Br3] (RI = 1.70). The quartz sample used was a rough crystal with six sides and many black inclusions. These inclusions are best seen in the nearly perfect match that is [C8mim]Br. The facets of the quartz crystal are starkly apparent in the fluids with the largest index mismatches, viz. in air and in [C4mim][Br3].The natural beryl and corundum samples are faceted, polished ovals, while the (artificial) cubic zirconia is a polished, faceted square shape (an “emerald cut”). The line of white dots near the middle of the beryl are small chips in the culet of that specimen, best seen in the [C8mim]Br image. Note that the corundum almost vanishes in the “best match” [C4mim][Br3], while its inclusions become very clear. The facets of the cubic zirconia become increasingly difficult to discern with fluids of increasing refractive index. The apparent “bending” of the edge of the cubic zirconia is due to surface tension of the water and ionic liquid near the side of the microbeaker which distorts the liquids’ surfaces.
Results and discussion
Synthesis and characterisation
Our original efforts to prepare high refractive index ionic liquids involved the preparation of salts containing 1-alkyl-3-methylimidazolium cations with hexahalostannate(IV) anions, [Cnmim]2[SnX6] (n = 6 or 8, X = Cl or Br), since the electron-rich tin(IV) anions were envisaged to give high refractive index products. Unfortunately, however, the tin(IV) salts exhibited melting points >150 °C, as anticipated by the Kapustinskii equation,26 rendering them unsuitable as high refractive index room temperature immersion liquids. The high melting points of the tin(IV) salts are in agreement with previous studies, which showed that imidazolium salts with dianionic metal halide counterions e.g. [NiCl4]2− or [CoCl4]2−,27 have higher melting points than analogous salts with singly-negative anions. Therefore, in order to keep the ionic liquid melting points low, whilst simultaneously attempting to achieve high refractive indices, ionic liquids with 1-alkyl-3-methylimidazolium cations and polyhalide anions were prepared (Table 1).Salts containing polyhalide anions have been known for many years and their preparation, solution-phase, and solid-state chemistry have been extensively investigated.28 The general method for the preparation of polyhalides involves direct combination of a halide salt, e.g. [C4mim]Br, and a halogen, e.g. I2, or interhalogen, e.g. IBr,28 the experimental conditions being dictated by the reactivity of the reactants and products. In the present study, an analogous approach was employed to synthesise the ionic liquids containing polyhalide anions.28,29 The more familiar ionic liquids, [C4mim][BF4], [C4mim][NTf2], [C4mim][OTf], [C4mim]Cl, [C4mim]Br, [C4mim]I, [C6mim]Br, [C6mim]I, and [C8mim]I were prepared using standard procedures (see Experimental section).
Microanalytical data (Table 2) confirmed the identity of the polyhalide anion ionic liquids, while the invariance of their refractive index data, which were regularly determined over a three year period (vide infra), demonstrated the stability of the fluids over time.
| Microanalysesa | ||||
|---|---|---|---|---|
| Ionic liquid | % C | % H | % N | Mp (°C) |
| a Required values given in parentheses. b Not determined for known samples. c Not observed. | ||||
| [C2mim][ClI2] | 18.01 (17.99) | 2.72 (2.77) | 7.40 (7.00) | —c |
| [C2mim][BrI2] | 16.25 (16.19) | 2.47 (2.49) | 6.50 (6.29) | 8.6 |
| [C2mim][IBr2] | 18.20 (18.11) | 2.62 (2.79) | 6.85 (7.04) | 3.9 |
| [C2mim][I7] | 7.34 (7.21) | 0.90 (1.11) | 2.86 (2.80) | —c |
| [C2mim][I9] | 5.95 (5.75) | 0.47 (0.88) | 2.24 (2.24) | —c |
| [C4mim][I3] | 18.83 (18.48) | 2.96 (2.90) | 5.58 (5.39) | —c |
| [C4mim][ClI2] | 22.44 (22.42) | 3.17 (3.53) | 6.45 (6.53) | —c |
| [C4mim][BrI2] | 20.17 (20.32) | 3.15 (3.19) | 6.04 (5.92) | —c |
| [C4mim][IBr2] | 21.61 (22.56) | 3.23 (3.54) | 6.13 (6.58) | —c |
| [C4mim][Br3] | 25.04 (25.34) | 3.88 (3.99) | 7.18 (7.39) | —c |
| [C4mim][I5] | 12.48 (12.42) | 2.03 (1.95) | 4.00 (3.62) | —c |
| [C4mim][I7] | 9.59 (9.35) | 1.27 (1.47) | 3.01 (2.73) | —c |
| [C6mim][I3] | 21.80 (21.92) | 3.13 (3.49) | 5.05 (5.11) | −9.8 |
| [C6mim]I | 39.78 (40.83) | 5.95 (6.51) | 9.23 (9.52) | —c |
| [C6mim][BrI2] | 23.01 (23.98) | 3.51 (3.82) | 5.12 (5.59) | —c |
| [C6mim][IBr2] | 25.64 (26.45) | 3.77 (4.22) | 5.79 (6.17) | —c |
| [C8mim]Br | —b | —b | —b | —c |
| [C4mim][BF4] | —b | —b | —b | —c |
| [C4mim][NTf2] | —b | —b | —b | —c |
| [C4mim][OTf] | —b | —b | —b | —c |
Differential scanning calorimetry (DSC) was employed to determine the melting points of the ionic liquids with polyhalide anions, but only [C2mim][IBr2], [C2mim][BrI2], and [C6mim][I3] displayed unambiguous melting transitions below 10 °C (Table 2). DSC scans of the remaining salts below 20 °C exhibited very broad transitions, if any, probably because the materials form glasses, a common feature of ionic liquids.13
The proton nuclear magnetic resonance (1H-NMR) spectra of the ionic liquids confirmed the structural assignments of the cations and also showed that halogenation of the imidazolium ring of the ionic liquids containing polyhalide anions had not occurred (Table 3), as is known to happen in reactions with dichlorine.30
| Ionic liquid | CH2 (s) | CH4 (s) | CH5 (s) | NCH2α (t or q) | NCH3 (s) | N(CH2)n−1CH3 (t) | NCH2(CH2)n−2 (m) | NC2H4CH2 (m) |
|---|---|---|---|---|---|---|---|---|
| a Recorded in d4-methanol. b Recorded in d3-ethanenitrile. c Recorded in d6-propanone. | ||||||||
| [C2mim][ClI2]c | 8.97 | 7.68 | 7.91 | 4.28 (q, J = 7.3) | 3.95 | 1.46 (J = 7.1) | — | — |
| [C2mim][BrI2]c | 8.95 | 7.58 | 7.65 | 4.43 (q, J = 7.5) | 3.96 | 1.57 (J = 7.5) | — | — |
| [C2mim][IBr2]c | 8.43 | 7.39 | 7.34 | 4.17 (q, J = 7.5) | 3.83 | 1.46 (J = 7.2) | — | — |
| [C2mim][I7]a | 8.92 | 7.63 | 7.83 | 4.24 (q, J = 7.3) | 4.00 | 1.54 (J = 7.1) | — | — |
| [C2mim][I9]a | 8.95 | 7.61 | 7.94 | 4.25 (q, J = 7.3) | 3.96 | 1.53 (J = 7.2) | — | — |
| [C4mim][I3]c | 9.04 | 7.62 | 7.75 | 4.25 (t, J = 7.2) | 3.98 | 0.85 (J = 7.2) | 1.86 (2H) | 1.26 |
| [C4mim][ClI2]c | 9.25 | 7.57 | 7.82 | 4.30 (t, J = 7.2) | 3.97 | 0.82 (J = 7.2) | 1.83 (2H) | 1.29 |
| [C4mim][BrI2]a | 8.94 | 7.56 | 7.63 | 4.24 (t, J = 7.5) | 3.95 | 0.99 (J = 7.2) | 1.89 (2H) | 1.39 |
| [C4mim][IBr2]b | 8.42 | 7.34 | 7.37 | 4.13 (t, J = 7.2) | 3.83 | 0.94 (J = 7.5) | 1.81 (2H) | 1.33 |
| [C4mim][Br3]c | 8.91 | 7.54 | 7.61 | 4.22 (t, J = 7.2) | 3.93 | 0.99 (J = 7.5) | 1.89 (2H) | 1.46 |
| [C4mim][I5]a | 8.94 | 7.58 | 7.64 | 4.24 (t, J = 7.2) | 3.96 | 1.00 (J = 7.2) | 1.91 (2H) | 1.40 |
| [C4mim][I7]a | 8.95 | 7.58 | 7.65 | 4.24 (t, J = 7.2) | 3.98 | 1.01 (J = 7.5) | 1.91 (2H) | 1.41 |
| [C6mim][I3]a | 8.94 | 7.65 | 7.58 | 4.24 (t, J = 7.2) | 3.96 | 0.92 (J = 6.9) | 1.38 (6H, Hγ,δ,ε); 1.91 (2H, Hβ) | — |
| [C6mim][IBr2]b | 8.40 | 7.37 | 7.33 | 4.11 (t, J = 7.2) | 3.82 | 0.88 (J = 6.3) | 1.30 (6H, Hγ,δ,ε); 1.83 (2H, Hβ) | — |
| [C6mim][BrI2]b | 8.59 | 7.62 | 7.41 | 4.17 (t, J = 7.2) | 3.90 | 0.95 (J = 7.1) | 1.30 (6H, m, Hγ,δ,ε); 1.96 (2H, m, Hβ) | — |
In line with previous observations, the colours of the polyiodide salts, [Cnmim][Ix] (x = 3, 5, 7, or 9), prepared in the current investigation become darker with increasing iodine content, with the [I3]− salts red and transparent, and the [I9]− salts exhibiting a characteristic metallic lustre.31 During the preparation of the [Ix]− (x = 3, 5, 7, or 9) salts, it was ensured that the corresponding precursor salts, [Cnmim]I, were extremely dry, since attempting to dry the polyiodide products in vacuo could result in their decomposition, evidenced by the visible liberation of iodine vapour. The observed iodine loss is in agreement with earlier studies, which have shown that polyhalide salts may thermally dissociate into free iodine and the precursor iodide salt.28
The solid state structures of many salts with polyiodide anions have shown that, with few exceptions, the structural entities can be divided into the fundamental and stable units I−, I2 and [I3]−.31 As illustrated by (a) and (b) in Fig. 3, the local structures of polyiodide salts can be rationalised in standard coordination chemistry terms where the ions, I− and [I3]− are ligated by neutral I2 molecules.
![Rationalisation of [I5]− anion structures; (a) [I5]−, first modification32 and (b) [I5]−, second modification.32](/image/article/2006/NJ/b513451j/b513451j-f3.gif) | ||
| Fig. 3 Rationalisation of [I5]− anion structures; (a) [I5]−, first modification32 and (b) [I5]−, second modification.32 | ||
The identity of the anions was confirmed by a combination of the liquid secondary ion (LSI) and electrospray ionisation (ESI) mass spectra (Table 4); the isotopic distributions of these ions containing chloride and bromide served as support for their identities. Both techniques required the use of the carrier solvents, 3-nitrobenzyl alcohol (noba) or ethanenitrile and methanol, respectively, which can affect the equilibria or nature of polyhalide anions.33 Indeed, a computational study performed by Sato et al.34 has suggested that the dissociation energy for [I3]− in ethanenitrile or methanol is smaller than in the gas state equilibrium structure, indicating that exchange reactions should proceed more easily in solution. Stated another way, the study implied that for solutions of [I3]−, the I2 constituent of the anion becomes isolated due to solvation and, therefore, dissociation of the anion is easier than in the gas state. Furthermore, the investigation by Sato et al.34 also suggested that the dissociation energy for [I3]− in methanol is smaller than in ethanenitrile, implying that anion dissociation should proceed more easily in methanol than ethanenitrile. In the current investigation, this phenomenon was observed in the negative mode electrospray ionisation mass spectra of ethanenitrile solutions of [C4mim][I3], and [C6mim][I3] (Table 4), i.e. signals for both [I3]− and I− were present.
| Ionic liquid | Method | Anionb | Additional mass-peaksb | |
|---|---|---|---|---|
| a m/z values refer to 79Br and 35Cl. b (m/z, relative intensity %). c Recorded in CH3CN. d Recorded at the EPSRC MS Centre in CH3OH. e Recorded at the EPSRC MS Centre in 3-nitrobenzyl alcohol. f Observed in the full scan ESI mass spectrum. g Observed in the positive mode ESI mass spectrum. h Observed in the full scan LSI mass spectrum. | ||||
| [C2mim][ClI2] | ESIc | [ClI2]− | [I3]− | [ICl2]− |
| (289; 9) | (381; 100) | (197; 3) | ||
| [C2mim][BrI2] | ESIc | [BrI2]− | [I3]− | [IBr2]− |
| (333; 27) | (381; 100) | (287; 6) | ||
| [C2mim][IBr2] | ESIc | [IBr2]− | [BrI2]− | [I3]− |
| (287; 100) | (335; 13) | (381; 5) | ||
| [C2mim][I7] | ESId | I− | — | — |
| (127; 100) | ||||
| [C2mim][I9] | LSIe | [I3]− | {[C2mim]I·4noba}− | {[C2mim]2I}− |
| (381; 44) | (863; 100) | (349; 22);h | ||
| {[C2mim]I2}− | ||||
| (365; 5)h | ||||
| [C4mim][ClI2] | ESIc | [ClI2]− | [I3]− | [ICl2]− |
| (289; 14) | (381; 100) | (197; 8) | ||
| [C4mim][BrI2] | ESIc | [BrI2]− | [I3]− | [IBr2]− |
| (333; 38) | (381; 100) | (287; 5) | ||
| [C4mim][IBr2] | ESIc | [IBr2]− | [BrI2]− | [I3]− |
| (287; 100) | (335; 4) | (381; 3) | ||
| [C4mim][I3] | ESIc | [I3]− | I− | {[C4mim]2I}− |
| (381; 100) | (127; 14) | (405; 8)f | ||
| [C4mim][I5] | LSIe | [I3]− | I− | {[C4mim]I2}− |
| (381; 100) | (127; 30) | (393; 21)h | ||
| [C4mim][I5] | ESIc | [I3]− | I− | {[C4mim]2I}− |
| (381; 100) | (127; 13) | (405; 100)g | ||
| [C4mim][I7] | ESId | I− | — | {[C4mim]I2}− |
| (127; 100) | (393; 18)h | |||
| [C6mim][BrI2] | ESIc | [BrI2]− | [I3]− | [IBr2]− |
| (333; 38) | (381; 100) | (287; 5) | ||
| [C6mim][IBr2] | ESIc | [IBr2]− | — | — |
| (287; 100) | ||||
| [C6mim][I3] | ESIc | [I3]− | I− | {[C6mim]2I}− |
| (381; 100) | (127; 20) | (461; 5);f | ||
| {[C6mim]I2}− | ||||
| (421; 21)f | ||||
To the best of our knowledge, no equilibrium data exist for higher polyiodides (> [I3]−) in ethanenitrile or methanol, but a similar dissociation mechanism to that of [I3]− anions is probable, since the local structures of polyiodide anions may be rationalised in standard coordination chemistry terms (Fig. 3). This postulate is supported by the negative mode electrospray ionisation scans of [C4mim][I5], recorded using ethanenitrile as infusion solvent, since signals for the intact anions are not observed; the only detectable anion peaks are [I3]− and I− (Table 4).
Liquid secondary ion mass full scan (both positive and negative) spectral data were also recorded for [C4mim][I5] in 3-nitrobenzyl alcohol, which showed strong signals for I− and [I3]−, but the remainder of the spectrum provided no conclusive structural information. The electrospray ionisation mass spectra of [C2mim][I7] and [C4mim][I7] recorded in methanol only exhibited strong signals for I−, while the full scan LSIMS mass spectra were too complex to allow any definite conclusions other than rearrangement possible under the experimental conditions.
Recent ab initio calculations have shown that mixed halide anions, [X–Y–X]−, where Y is heavier than X, are more stable than [Y–X–X]− isomers, both in the gas phase and in solution.35 As a result, [Y–X–X]− anions, if formed from X2 and Y−, are expected to isomerise12 to [X–Y–X]− anions via cleavage of the X–X bond. This phenomenon was observed in the mass spectra of [C2mim][ClI2], [C2mim][BrI2], [C4mim][ClI2], [C4mim][BrI2], [C6mim][IBr2], and [C6mim][BrI2], where each of the analysed compounds (Table 4) exhibited the expected anion fragment together with additional species formed by disproportionation, eqn (2).
| 2[YX2]− ⇌ [X3]− + [Y2X]− | (2) |
Refractive index measurements
The refractive indices of the polyhalide ionic liquids were measured using either optical microscopy or interferometry (Table 5). The method employed to measure refractive indices depended on the appearance of the ionic liquid; the refractive indices of the transparent ionic liquids were measured using standard optical microscopy methods, viz. (i) Becke’s method22 or (ii) using a depth-measuring microscope,2 while a Fabry–Perot interferometer24 was employed to determine the refractive indices of the intensely coloured salts.| Ionic liquid | R I a | R I b | R I c |
|---|---|---|---|
| a Becke’s method,22 2002, estimated error ± 0.005. b Depth-measuring microscope,2 2003, estimated error: ± 0.002. c Fabry–Perot interferometer,24 2003, estimated error: ± 0.001. d Fabry–Perot interferometer,24 2004, estimated error: ± 0.001. | |||
| [C2mim][ClI2] | 1.796 | — | — |
| [C2mim][BrI2] | 1.833 | 1.833 | — |
| [C2mim][IBr2] | — | 1.715 | — |
| [C2mim][I7] | — | — | 2.010d |
| [C2mim][I9] | — | — | 2.080d |
| [C4mim][I3] | — | — | 1.700 |
| [C4mim][ClI2] | 1.774 | 1.775 | — |
| [C4mim][BrI2] | 1.810 | — | 1.805 |
| [C4mim][IBr2] | — | 1.701 | — |
| [C4mim][Br3] | 1.67 | 1.699 | — |
| [C4mim][I5] | — | — | 1.910 |
| [C4mim][I7] | — | — | 1.950 |
| [C6mim][I3] | — | — | 1.880 |
| [C6mim]I | 1.549 | — | 1.547 |
| [C6mim][BrI2] | 1.768 | — | — |
| [C6mim][IBr2] | — | 1.685 | — |
| [C8mim]Br | 1.490 | — | — |
| [C4mim][BF4] | 1.416 | 1.410 | — |
| [C4mim][NTf2] | 1.451 | — | — |
| [C4mim][OTf] | 1.433 | — | — |
The refractive index data indicate that the values remained essentially the same over a period of at least one year, which is encouraging for the storage of these ionic liquids as stable immersion fluids for optical mineralogical studies (Table 5). The refractive index measurements also show the reproducibility of the data, since three techniques were employed and only minor discrepancies exist.
Metathesis, or anion exchange, is one of the most common routes to prepare ionic liquids, shown in eqn (3) for the preparation of [Cnmim][BF4] salts.36 As a result, ionic liquids can contain large amounts of chloride if not removed by washing the product with water, which is also a contaminant if not removed. Such impurities are known to affect many physical properties of ionic liquids,37 including refractive index, as shown in this work.
 | (3) |
Refractive index data of ionic liquids are currently limited, with only a few values reported in the literature and none in the most recent data compilation.6 Since ionic liquid properties are acutely affected by the presence of impurities, especially chloride and/or water, it is essential that purity information always accompanies physical property data (Table 6). For example, Kim et al.38 give the refractive index of [C4mim][BF4] as 1.4227, but provide no purity information for the ionic liquid, minimizing the value of the published refractive index. In contrast, Sigma-Aldrich39 reports the refractive index of the same salt as 1.42 and the chloride and water contents as <10 mg kg−1 and <0.02% (w/w), respectively, which are very similar to the 12.6 mg kg−1 chloride and 0.03% (w/w) water contents of our sample (Table 6). Therefore, if Kim et al.’s38 refractive index value is disregarded, the standard deviations of the refractive index values of our [C4mim][BF4] sample, and that of Sigma-Aldrich, ±0.01, lie within experimental error of each other and thus are essentially the same.
| Ionic liquid | Source | R I | Cl− (mg kg−1) | H2O (% w/w) | Other impurities (mg kg−1) |
|---|---|---|---|---|---|
| a Sigma-Aldrich 2004–2005 Catalogue. b Purity: 98.5% (NMR). c Not reported. d Purity: 98% (NMR). e Purity: 95% (NMR). | |||||
| [C4mim][BF4] | QUILL | 1.410 | 12.6 | 0.03 | 0 |
| Sigma-Aldricha,b | 1.42 | <10.0 | <0.02 | 15 000 | |
| Kim et al.38 | 1.4227 | —c | —c | —c | |
| [C4mim][NTf2] | QUILL | 1.451 | ca. < 5 | 0.01 | 0 |
| Sigma-Aldricha,d | 1.428 | —c | <0.5 | 2000 | |
| [C4mim][OTf] | Bonhôte et al.15 | 1.4271 | —c | 1.4 | —c |
| QUILL | 1.433 | <7 | 0.03 | 0 | |
| Sigma-Aldricha,e | 1.434 | —c | —c | 5000 | |
| Bonhôte et al.15 | 1.4380 | —c | —c | —c | |
Considering the data and purity information given in Table 6 for [C4mim][NTf2] and [C4mim][OTf] from various sources, it is again clear that water and chloride affect the refractive indices of ionic liquids. It must be mentioned here that at present, there is no published, standard method to determine the chloride content of [NTf2]− ionic liquids, although preliminary ion chromatography studies in our laboratories indicate that chloride contents of the salts can be determined at levels of ca. <5 mg kg−1.40
Fig. 4 is a plot of the refractive indices for all the investigated ionic liquids as a function of their total, anion and cation formula weights (FW). As expected, the salts containing the highest electron density anions viz. [C2mim][I7], [C2mim][I9] and [C4mim][I7], exhibit the highest refractive indices; 2.01, 2.08, and 1.95, respectively. Fig. 4 also indicates that the ionic liquid refractive indices are governed by a primary anion and secondary cation effect, i.e. the refractive indices of the compounds increase as the anion mass increases provided the 1-alkyl chain length of the cation remains short.
 | ||
| Fig. 4 Plot of refractive indices versus cation, anion, and total formula weights of the investigated ionic liquids. | ||
The effect that shorter 1-alkyl chains have on the refractive indices of a series of salts with the same anion is illustrated in Fig. 5, which shows how the refractive indices of the [IBr2]− and [BrI2]− salts systematically decrease by 0.02 and 0.03 units, respectively, as the 1-alkyl chain length on the cations increases monotonically. Regression analyses of the refractive index vs. cation formula weights plots for the [IBr2]− and [BrI2]− salts (Fig. 5), gave respective coefficient of determination, R2, values of 0.9986 and 0.9938, indicating their potential application in predicting refractive indices of ionic liquids with the same anion.
![Refractive indices versus cation, anion, and total formula weights of the [Cnmim][IBr2] and [Cnmim][BrI2] (n = 2, 4, or 6) ionic liquids.](/image/article/2006/NJ/b513451j/b513451j-f5.gif) | ||
| Fig. 5 Refractive indices versus cation, anion, and total formula weights of the [Cnmim][IBr2] and [Cnmim][BrI2] (n = 2, 4, or 6) ionic liquids. | ||
Similarly to the [IBr2]− ionic liquids, the salts with [C2mim]+ and [C4mim]+ cations and [I3]−, [I7]−, [ClI2]−, and [BrI2]− anions, exhibit refractive index increases that coincide with shortening the 1-alkyl chains (Table 5). However, a regular relationship between refractive index and anion mass is not observed for a series of ionic liquids with the same cation but different anions, viz. [C4mim]X (X = [I3], [I5] and [I7]), (Table 5). A large refractive index difference exists between [C4mim]I38 and [C4mim][I3] (ΔRI = 0.131), as well as between [C4mim][I3] and [I5] (ΔRI = 0.210), but the difference is much smaller between [C4mim][I5] and [I7] (ΔRI = 0.040). There were two possible explanations for this unexpectedly small change. Either (a) the philosophy that we had developed was incorrect or (b) the anion we had anticipated was not present. As polyhalides, in general, and polyiodides in particular, are known to dissociate according to eqn (4), we deliberately added an excess of I2 to the solution to force the equilibrium to the left. In the case of the anticipated [I7]− system, for example, this increased the observed value of RI from 1.95 to 2.01, reinforcing the design principle. Interestingly, an early 1903 RI value for liquid I2 was given as close to 2.08.41
| [In]− ⇌ [In−2]− + I2 | (4) |
![(a) Refractive index and (b) density vs. anion FW of [C4mim][Ix] (x = 1, 3, 5, or 7) salts ( = measured values; = predicted value; = saturated I2 solution).](/image/article/2006/NJ/b513451j/b513451j-f6.gif) | ||
Fig. 6 (a) Refractive index and (b) density vs. anion FW of [C4mim][Ix] (x = 1, 3, 5, or 7) salts ( = measured values; = measured values;  = predicted value; = predicted value;  = saturated I2 solution). = saturated I2 solution). | ||
Refractive indices and inclusions in gems and minerals
The key aim of the current work was to design and test ionic liquids as immersion fluids for optical mineralogy studies that are more environmentally benign than many materials that are currently used for this purpose. As already mentioned, inclusions in minerals are of great interest to earth scientists since they provide important clues about the conditions at the time of mineral formation. Refractive indices often uniquely identify a mineral. These are typically determined using Becke’s method,22 which involves immersing a mineral in a fluid with a comparable refractive index, and examining the immersed mineral under a microscope. Under these conditions, the mineral virtually ‘disappears’ under darkfield microscope illumination, which eliminates light reflection from the mineral’s surface, concurrently highlighting inclusions therein. Using this approach, we studied four minerals viz. colourless quartz, orange beryl, brown corundum, and colourless cubic zirconia (synthetic), with respective refractive indices of 1.54, 1.59, 1.76 and 2.16, by immersing them in air, water, [C8mim]Br, and [C4mim][Br3], with refractive indices of 1.00, 1.33, 1.49, and 1.70, respectively. The quartz sample used was a rough crystal with six sides and many black inclusions. The natural beryl and corundum samples are faceted, polished ovals, while the (artificial) cubic zirconia is a polished, faceted square shape (an “emerald cut”).Fig. 7 shows the images obtained for the four investigated minerals in (a) air and (b) water. In Fig. 7(a), the facets of the quartz, cubic zirconia, and orange beryl crystals are plainly visible due to extensive light reflection from their surfaces. The apparent “bending” of the edge of the cubic zirconia is due to surface tension of the water, Fig. 7(b), and ionic liquid, Fig. 8(a), near the side of the microbeaker, which distorts the liquids’ surfaces.
 | ||
| Fig. 7 Images of quartz, orange beryl, brown corundum, and colourless cubic zirconia immersed in (a) air and (b) water. | ||
In Fig. 7(b), the facets of the minerals become less discernable since their immersion in water results in decreased light reflection from their surfaces, i.e. the facets of the sample crystals become increasingly difficult to discern with fluids of increasing refractive index. A refractive index match between the minerals and their immersion media is not evident in Fig. 7(a) or 7(b) due to the large refractive index mismatches between the minerals and their host media.
In contrast to Fig. 7(a) and 7(b), in Fig. 8(a), a close refractive index match between [C8mim]Br (RI = 1.49) and quartz (RI = 1.54), is evidenced by the almost invisible appearance of the quartz crystal, which is only discernable by its black inclusions.
![Images of quartz, orange beryl, brown corundum, and colourless cubic zirconia immersed in (a) [C8mim]Br, and (b) [C4mim][Br3].](/image/article/2006/NJ/b513451j/b513451j-f8.gif) | ||
| Fig. 8 Images of quartz, orange beryl, brown corundum, and colourless cubic zirconia immersed in (a) [C8mim]Br, and (b) [C4mim][Br3]. | ||
In Fig. 8(b), a refractive index match is again visible, this time between brown corundum (RI = 1.76) and [C4mim][Br3] (RI = 1.70), and where only the inclusion in the brown corundum remains visible. Fig. 8(a) and 8(b) represent typical examples of how earth scientists could locate inclusions in unpolished minerals without encumbrance by light reflection. An existing alternative immersion medium to [C4mim][Br3] is toxic diiodomethane saturated with sulfur (RI = 1.78). The odourless, transparent ionic liquid (with negligible vapour pressure) has a major health and safety advantage over the traditional fluid. Although the ionic liquids investigated with RI > 1.8 are opaque to the naked eye, they do transmit near infrared radiation; we thus continue our search for materials with RI > 1.8 that are transparent in the infrared.
Conclusion
One of the most attractive features of ionic liquids for scientists and engineers is their potential to be tailored for predetermined function. In the present study, the potential to prepare ionic liquids with specific refractive indices is demonstrated. The prediction of these values, by application of the parachor, is described elsewhere.42Although we only studied about 20 examples of neoteric fluids, we have elaborated a design principle which will enable these fluids to be prepared with RI in the range 1.40–2.10, at intervals of 0.01 (either pure liquids or binary systems). Twenty ionic liquids mixed in binary fashion generate 400 novel immersion media, even with simple 1∶1 ratios. If ratios are changed in steps of 0.1, then 4000 new liquids will be generated.
In this work, we have potentially increased the number of RI matching media by approximately 700 liquids. As these fluids are ionic liquids, they have negligible vapour pressure, and, by virtue of this physical property, are more environmentally benign than the vast majority of existing high refractive index immersion fluids for optical mineralogy. In this work, we have thus not only made refractive index measurements more precise, but have also substantially reduced health risks to the experimental scientist.
Acknowledgements
We gratefully acknowledge the United Institute of Geology, Geophysics & Mineralogy (UIGGM), Siberian Branch of the Russian Academy of Sciences at Novosibirsk for the use of the photograph shown in Fig. 1. MD thanks QUILL and its industrial advisory board for funding, and MS thanks J. Webster, C. Mandeville and R. Fogel for discussions and access to their American Museum of Natural History laboratories. We thank David Shara for the loan of mineral specimens and for technical assistance in producing the images of those minerals in air, water and ionic liquids. We also gratefully acknowledge the EPSRC National Mass Spectrometry Service Centre at the University of Wales, Swansea for recording mass spectra. MD and KRS also gratefully acknowledge the EPSRC for funding under their Portfolio Partnership Scheme (Grant no. EP/D029538/1).References
- IUPAC Compendium of Chemical Technology, web: http://www.iupac.org/goldbook/R05240.pdf Search PubMed.
- M. Born and E. Wolf, Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light, Cambridge University Press, London, 7th edn, 1999 Search PubMed.
- American Museum of Natural History. The Nature of Diamonds, web: http://www.amnh.org/exhibitions/diamonds/ Search PubMed.
- Handbook of Chemistry and Physics, ed. D. R. Lide, CRC Press, London, 75th edn, 1994 Search PubMed.
- V. Mallette, INWIT™ - Refractive Indices, web: http://www.inwit.com/inwit/writings/refractiveindices.html Search PubMed.
- Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, 2003 Search PubMed.
- (a) Ionic Liquids IIIA: Fundamentals, Progress, Challenges, and Opportunities, ACS Symp. Ser., ed. K. R. Seddon and R. D. Rogers, American Chemical Society, Washington DC, 2005, vol. 901 Search PubMed; (b) Ionic Liquids IIIB: Fundamentals, Progress, Challenges, and Opportunities - Transformations and Processes, ACS Symp. Ser., ed. K. R. Seddon and R. D. Rogers, American Chemical Society, Washington DC, 2005, vol. 902 Search PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772 CrossRef.
- M. Freemantle, Chem. Eng. News, 1998, 76, 32.
- M. S. Selvan, M. D. McKinley, R. H. Dubois and J. L. Atwood, J. Chem. Eng. Data, 2000, 45, 841 CrossRef CAS.
- O. Bortolini, M. Bottai, C. Chiappe, V. Contec and D. Pieraccini, Green Chem., 2002, 4, 621 RSC.
- J. D. Holbrey and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2133 RSC.
- C. P. Fredlake, J. M. Crosthwaite, D. G. Hert, S. N. V. Aki and J. F. Brennecke, J. Chem. Eng. Data, 2004, 49, 954 CrossRef CAS.
- P. Bonhôte, A.-P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Inorg. Chem., 1996, 35, 1168 CrossRef CAS.
- M. Deetlefs and K. R. Seddon, Green Chem., 2003, 5, 181 RSC.
- EPSRC National Mass Spectrometry Service Centre, web: http://www.swan.ac.uk/nmssc Search PubMed.
- MARS Synthesis: Combinatorial and Parallel Synthesis Workstation, web: http://www.cemsynthesis.com/ Search PubMed.
- C. Villagrán, M. Deetlefs, W. R. Pitner and C. Hardacre, Anal. Chem., 2004, 76, 2118 CrossRef CAS.
- K. R. Seddon, A. Stark and M.-J. Torres, Pure Appl. Chem., 2000, 72, 2275 CrossRef CAS.
- A Textbook of Quantitative Inorganic Analysis, ed. A. I. Vogel, Longmans, Green and Co., London, 3rd edn, 1961 Search PubMed.
- F. Becke, Sitzungsber. Kais. Akad. Wiss. Wein, Math. Naturwiss. Kl., Abt. 1, 1893, 102, 358 Search PubMed.
- Cargille Laboratories Reference Standards, web: http://www.cargille.com/referencestandards.shtml Search PubMed.
- J. M. Vaughan, The Fabry–Perot Interferometer: History, Theory, Practice, and Applications, Institute of Physics Publishing, Bristol, 1989 Search PubMed.
- P. W. Atkins, Physical Chemistry, Oxford University Press, Oxford, 5th edn, 1994, p. 734 Search PubMed.
- A. F. Kapustinskii, Q. Rev. Chem. Soc., 1956, 10, 283 RSC.
- P. B. Hitchcock, K. R. Seddon and T. Welton, J. Chem. Soc., Dalton Trans., 1993, 2639 RSC.
- A. J. Downs and C. J. Adams, in Comprehensive Inorganic Chemistry, ed. J. C. Bailar, H. J. Emeléus, R. Nyholm and A. F. Trotman-Dickenson, Pergamon Press Ltd, Oxford, 1975, vol. 3, p. 1534 Search PubMed.
- P. H. Svensson and L. Kloo, J. Chem. Soc., Dalton Trans., 2000, 14, 2449 RSC.
- N. Winterton, K. R. Seddon and Y. Patell, World Patent, WO 0037400, 2000 Search PubMed.
- P. H. Svensson and L. Kloo, Chem. Rev., 2003, 103, 1649 CrossRef CAS.
- F. H. Herbstein and M. Kapon, Nature, 1972, 239, 153 CAS.
- N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Pergamon Press Ltd, Oxford, 1984, pp. 978–983 Search PubMed.
- H. Sato, F. Hirata and A. B. Meyers, J. Phys. Chem. A, 1998, 102, 2065 CrossRef CAS.
- Y. Ogawa, O. Takanashi and O. Kikuchi, J. Mol. Struct., 1998, 429, 187 CAS.
- A. J. Carmichael, M. Deetlefs, M. J. Earle, U. Fröhlich and K. R. Seddon, in Ionic Liquids as Green Solvents: Progress and Prospects, ACS Symp. Ser., ed. K. R. Seddon and R. D. Rogers, American Chemical Society, Washington DC, 2005, vol. 856, p. 14 Search PubMed.
- K. R. Seddon, A. Stark and M. J. Torres, Pure Appl. Chem., 2000, 72, 2275 CrossRef CAS.
- K.-S. Kim, B.-K. Shin and H. Lee, Korean J. Chem. Eng., 2004, 21, 1010 Search PubMed.
- Sigma-Aldrich, web: http://www.sigma-aldrich.com Search PubMed.
- G. Driver and K. R. Seddon, unpublished data.
- W. W. Coblentz, Phys. Rev., 1903, 16, 72.
- M. Deetlefs, K. R. Seddon and M. Shara, Phys. Chem. Chem. Phys., 2006, 8, 262 Search PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2006 |