Hot off the Press
In the Hot off the Press section of Molecular BioSystems members of the Editorial Board and their research groups highlight recent literature for the benefit of the community. This month the highlighted topics include the cytotoxic effect of nanotubes, a method for simultaneous multiple biomarker detection and new evidence that clathrin polymerization causes endocytosis.
Toxic effects of carbon-based nanomaterials
Carbon based nanomaterials are attracting a significant amount of interest, particularly because of their anticipated use in biomedicine and nanoelectronics. Rapid progress has also been achieved in the chemical modification of carbon nanotubes or fullerenes to make them suitable for the delivery of DNA, proteins or drugs to the cells. However, the studies of cytotoxic effects of those nanomaterials are rare and not often conclusive. A group of Swiss scientists has tackled this problem in a recent paper.Structural similarity between asbestos (a highly toxic fibrous form of a normally benign silicate mineral) and carbon nanotubes (CNT) raised the concerns about the potential biotoxicity of widely used single and multi walled CNTs. Therefore, the systematic in vitro study with different carbon nanomaterials (nanotubes, nanofibres and flake like shaped carbon nanoparticles) was performed using human tumor cell line H596. The cytotoxicity was evaluated by MTT assay, where formation of formazan crystals in living cells (but not in dead ones) can be used to quantify the number of living cells. The toxic effects of carbon nanomaterials (0.02 μg ml−1) were noticed already within 24 h of exposure and the number of viable cells decreased as a function of concentration. Microscopic examination of the cells showed retraction of cytoplasm and decrease of nuclei size which is typical for irreversible cell injury and cell death (Fig. 1). Since chemically modified CNTs are often used for DNA and protein binding and drug transport, the results of their effect on cell proliferation were particularly important. A range of carboxyl, carbonyl and hydroxyl modified CNTs were prepared and significant reduction in the number of viable cells was observed. Although the exact mechanism leading to cell death is sill not known, the results clearly showed that more emphasis should be put on the cell toxicity study of these materials. Further work in that direction is particularly important in cases where they are used in biomedical applications like drug delivery or MRI screenings.
 | ||
| Fig. 1 The effect of CNTs on cells. (a) A typical control image of H596 cells vs. (b) H596 cells after 1 day's treatment. Cells have lost their mutual attachments, retracted their cytoplasm (arrows), and the nuclei are smaller. Reprinted with permission from Nano Lett., 2006, volume 6, issue 6, pages 1121–1125. Copyright 2006 American Chemical Society. | ||
A. Magrez, S. Kasas, V. Salicio, N. Pasquier, J. W. Seo, M. Celio, S. Catsicas, B. Schwaller, L. Forro, Cellular Toxicity of Carbon-Based Nanomaterials, Nano Lett., 2006, 6(6), 1121–1125.
Reviewed by: Ljiljana Fruk, Universität, Dortmund, GermanySimultaneous multiple biomarker detection
Advances in proteomics have led to the discovery of new potential disease-state biomarkers. These molecular markers could be employed, along with previously known markers, to provide more accurate medical diagnosis and prognosis, particularly in complex diseases like cancer. However, a disconnection exists between the emphasis in discovering new biomarkers and the practical application of these developments in a clinical setting. Currently, clinics rely heavily on Enzyme-Linked Immunosorbent Assays (ELISAs) to detect protein markers. While ELISAs can be reliably performed by a trained technician for single antigens, simultaneous analysis for multiple markers is not trivial. Developing a highly sensitive, selective, multiplexing ELISA platform that is both robust and high throughput is key to harnessing the full technological and proteomic advances of the last few years. Mirkin and coworkers recently unveiled a first step toward this goal. The group's strategy for multiplexed detection of protein markers uses two different metal particles which have unique physical properties that allow for easy enrichment, high selectivity, signal amplification, and excellent multiplexing opportunities. The first step of the assay is to add magnetic particles functionalized with antibodies against the biomarker of interest to the sample. The resulting magnetic particle–biomarker complexes are then labeled with a second particle—a “biobarcode,” which consists of a gold nanoparticle functionalized with both an antibody against a different epitope of the biomarker, as well as multiple copies of an oligonucleotide identity tag. The biomarker–particle complexes can then be readily isolated by simply placing the sample in an electric field and washing away the residual sample and biobarcodes. After this purification, the oligonucleotide identity tags on the biobarcodes are chemically cleaved and allowed to hybridize to complementary sequences on a DNA microarray. Addition of a universal detecting reagent, followed by scanometric detection of the resulting signal, provides sensitive detection of the original biomarker (Fig. 2). In this proof of principal paper, they demonstrate the assay for the simultaneous detection of three known cancer markers, prostate specific antigen, human chorionic gonadotropin, and alpha fetoprotein, in low pM concentrations in undiluted serum and 170 fM in diluted serum. However, because many oligonucleotide tags are available, an almost unlimited number of biomarkers could theoretically be detected in parallel. In addition, the ability to amplify the signal (due to the multiple oligonucleotide identity tags released per biomarker), and the assay's high selectivity (biomarkers are only detected if both particles' antibodies bind to them, minimizing cross-reacting signals), the group has made a significant advance towards highly multiplexed sensitive and selective biomarker detection.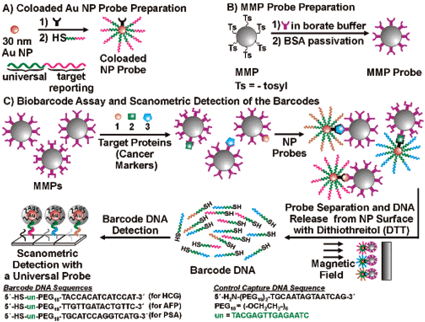 | ||
| Fig. 2 Biobarcode Assay for Multiplexed Protein Detection. Reprinted with permission from J. Am. Chem. Soc., 2006, 128(26), 8378–8379. Copyright 2006 American Chemical Society. | ||
S. I. Stoeva, J.-S Lee, J. E. Smith, S. T. Rosen, C. A. Mirkin, J. Am. Chem. Soc., 2006, 128(26), 8378–8379.
Reviewed by: Cleo Salisbury, Scripps Research Institute, USANo clathrin, no curvature
Endocytosis at the cell plasma membrane occurs mostly via budding of small clathrin coated vesicles. It is widely accepted that membrane deformation is induced by clathrin polymerization. This hypothesis has been proposed more than 30 years ago, and is mostly based on in vitro experiments showing that clathrin can self-assemble to form closed cages of the same diameter as clathrin coated vesicles (60–150 nm). Surprisingly, a clear demonstration that clathrin is required for budding has not yet been provided. Lars Hinrichsen, Ernst Ungewickell and co-workers now show that the clathrin adaptor protein AP2 forms small size domains (10–15 nm) on the plasma membrane independently of clathrin assembly. In the absence of clathrin, these AP2 domains remain flat, whereas they curve and invaginate to form clathrin coated vesicles in the presence of clathrin (Fig. 3). The authors propose a model in which the formation of clathrin coated buds is initially driven by spontaneous thermal fluctuations of flat clathrin lattices. Because clathrin self-assembles in highly curved structures, fluctuations with inward curvature may be trapped and amplified by clathrin polymerization to form a coated bud. These interesting results and ideas clearly point to a key role for clathrin in bud formation. However, they are derived from static observations made by immunofluorescence in fixed cells and by electron microscopy. A dynamic view of how clathrin bends a membrane is thus still lacking.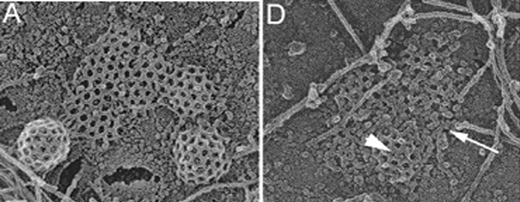 | ||
| Fig. 3 Control cells show numerous clathrin coated buds and flat lattices in electron microscopy (A). Clathrin-depleted cells are characterized by incomplete clathrin lattices (arrowhead in D) and uncoated flat clusters (arrow in D) that can be identified as AP2 domains by immunogold labelling. Figure taken from Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8715–8720. Copyright 2006 National Academy of Sciences, U.S.A. | ||
L. Hinrichsen, A. Meyerholz, S. Groos and E. J. Ungewickell, Bending a membrane: how clathrin affects budding, Proc. Natl. Acad. Sci. USA, 2006, 103, 8715–8720
Reviewed by: Jean-Baptiste Manneville, CNRS-Institut Curie, Paris, FranceDynein–dynactin has it both ways
The microtubule network provides an infrastructure for the movement of cytoskeletal motor proteins, which can move along them using ATP. Motor proteins recognize a structural asymmetry in the microtubules, which have a so-called plus-end and a minus-end. It is generally assumed that the motor kinesin moves in the plus-end direction, whereas dynein moves exclusively in the minus-end direction. Together these motors move a variety of cargo, notably vesicles, and an important way to form a link between the motors and the cargo is through the multi-functional protein complex dynactin.Using purified GFP-labeled dynein–dynactin complexes, Ross and Holzbauer and coworkers studied the movement of single dynein–dynactin complexes on microtubules in vitro (Fig. 4). They demonstrate that such single dynein–dynactin complexes (but not an ensemble of complexes attached to one single bead) can switch from plus-end directed to minus-end directed motion and vice versa. The authors observe relatively long-distance bi-directional movement, with a velocity that depends on the ATP concentration, suggesting that it is a real, non-diffusive, characteristic of the dynein–dynactin complex.
In vivo, a multitude of cargo is observed to move bi-directionally, and generally this is explained by plus-end and minus-end directed motors attaching to the same cargo. These new results suggest, however, that “just” the dynein–dynactin complex might drive some of this bi-directional movement.
 | ||
| Fig. 4 TIRF-microscopy of a dynein–dynactin–GFP that moves on a microtubule (not visible in b–h) and reverses direction. Reprinted by permission from Macmillan Publishers Ltd: Nat. Cell Biol., 2006, 8, 562–570, copyright 2006. | ||
J. L. Ross, K. Wallace, H. Shuman, Y. E. Goldman, and E. L. F. Holzbaur, Nat. Cell Biol., 2006, 8, 562–570
Reviewed by: Gerbrand Koster, Lab. PhysicoChimie Curie, Institut Curie, Paris, France| This journal is © The Royal Society of Chemistry 2006 |
