Disposable real-time microPCR device: lab-on-a-chip at a low cost
Pavel
Neuzil
*,
Juergen
Pipper
and
Tseng Ming
Hsieh
Institute of Bioengineering and Nanotechnology, 31 Biopolis Way, Nanos #04-01, Singapore 138669. E-mail: pneuzil@ibn.a-star.edu.sg
First published on 17th May 2006
Abstract
We have designed, fabricated and tested a real-time micro polymerase chain reaction (microPCR) system. It consists of a microscope glass cover slip placed on top of a micromachined silicon chip integrated with a heater and a temperature sensor. A single μL of a sample containing DNA was placed on the glass and encapsulated with mineral oil to prevent the evaporation of water, thus forming a virtual reaction chamber (VRC). The PCR chip required half a second to heat up from 72 to 94 °C and two seconds to cool from 94 to 55 °C, corresponding to a cooling rate of −20 K s−1. The real-time PCR yield was determined by a fluorescence method. The melting curve analysis method as well as capillary electrophoresis was performed to determine the purity of the PCR product. As the glass slip is disposable, cross-contamination from sample to sample is eliminated. The total cost of running the PCR is given by the value of the cover slip and its treatment.
Introduction
Since its original introduction,1 the polymerase chain reaction (PCR) has become the method of choice to multiply the number of specific DNA segments in a sample. The typical three step PCR cycle consists of heating up the sample from 90 to 94 °C to denature double-stranded DNA, cooling down to 50–70 °C to renature the specific primers to the single stranded DNA (annealing) and finally increasing the temperature to 70–75 °C for extension of the primers with thermostable DNA polymerase (elongation).2 Commercially available PCR equipments (e.g. MJ Research, Inc.) perform PCR simultaneously in 96 or more plastic tubes or wells containing sample DNA and PCR mixtures.It is important that the PCR system3 has good temperature control, maintains temperature uniformity within the sample and has a sample heating (cooling) rate of at least 5 K s−1 (−5 K s−1). Sample to sample cross-contamination should be also avoided. Temperature control is typically performed by a feedback loop system, while temperature uniformity is achieved by highly thermally conductive but bulky materials such as copper. A high heating rate is accomplished by the implementation of a proportional integrated derivative (PID) control method limited by maximum dissipated power and heat capacitance. A high cooling rate is rather difficult to achieve and bulky systems require forced cooling by either a thermoelectric element4 (often called a Peltier element) or by other means, such as water.5 They end up being complicated and even more power hungry devices than conventional PCR systems.
As the systems are bulky, their thermal time constants are in minutes rather than seconds. That results in long transition times and unwanted by-products of the PCR. The high power consumption eliminates the possibility of making a battery-operated and portable PCR system. In addition, the reaction tubes are large and the required amount of PCR cocktail makes the whole process expensive. Also, the detection of PCR product has to be done off-line, i.e. in another instrument, resulting in additional cost.
Current trends in μPCR technology
With the recent advancement of silicon technology based micromachining6 and biological micro-electromechanical systems (bioMEMS),7 many groups around the world have started the development of microPCRs (μPCR),8 which are expected to become a central part of a lab-on-a-chip9 or micro total analysis systems (μTAS). Researchers follow two basic approaches: a stationary system with cycling temperature10 or a flow system with three zones at different temperatures.11,12 Both systems have their advantages and disadvantages. Stationary systems cycle the temperature of the chamber in order to modify the temperature of the PCR solution. They do not require a pumping system or other means to move the PCR sample around. The flow-through systems typically have zones at three constant temperatures. Only the sample changes temperature by moving between zones of different temperatures. This type of PCR system is faster than the first one but it requires an implementation of a mechanism to move the sample around. In both cases, the heaters are integrated with the PCR system, so it is not economical to dispose the device to avoid cross-contamination after performing only a single test.µPCRs systems can be also categorized based on the heating system, which is either direct or indirect. Direct heating PCRs have the heater as well as the temperature sensor integrated with the device. Indirect heating can be performed by infrared radiation.13 PCR chips with integrated heaters/sensors are more complicated that ones with indirect heating but overall, the whole system is more compact and better-suited for small, portable PCR systems.
Currently, the three most popular materials for μPCR fabrication are silicon, glass and plastic. Silicon is an excellent material for a thermal-cycler chamber. It has a high thermal conductivity λ of 157 W m−1 K−1 and once it is thermally isolated, the chamber has good thermal uniformity.10 Also, micromachining is well established for this material. The drawback is that the silicon surface itself inhibits the PCR and its surface has to be covered with another material, such as silicon oxide or silicon oxynitride.
There have been attempts to make PCR chips on a glass substrate with a thin14 or thick film metal heater and sensor. Glass has a thermal conductivity λ of 1.1 W m−1 K−1, more than a hundred times lower than that of silicon. Due to its low thermal conductance, the systems made of glass are thermally isolated. However, creating a microfluidic system by glass machining is rather difficult compared to either silicon or plastic processing.
The obvious solution is to use micromachined silicon capped with another chip, which can be made of either silicon or glass.15 A short response time and low power consumption was achieved with systems made of both materials. The drawback is that the micromachining technique is relatively complicated and consists of 4–6 levels of lithography, including silicon etching and wafer to wafer bonding. Nevertheless, the combination of glass and micromachined silicon gives the designer two materials with a large difference in their thermal conductance, which allows tailoring of thermal and mechanical properties.
The third material commonly used for PCR is plastic (such as polycarbonate),16 polydimethylsiloxane (PDMS)17 or a composite used for printed circuit boards (PCB).18 All those materials have a cost advantage over both silicon as well as glass and they are simple to process. Polycarbonate can be shaped by a hot embossing technique,16 while PDMS polymerises in a mold.17 PCB technology is also well established. The common drawback is the low thermal conductivity of the plastic, and thus it should be combined with either metal or silicon in order to achieve the desired thermal properties.
A simplified approach was recently19 proposed, where the reaction chamber was made by encapsulation of a water based sample in oil, forming a virtual reaction chamber (VRC). As no solid cover or microchannels were required, the device fabrication then consisted only of deposition and patterning thin film heaters and temperature sensors on a suitable substrate, which is still too costly for a disposable system.
In order to eliminate cross-contamination between samples, the safest way is by using a disposable system. At the very least, the part of the device, which comes into contact with the sample should be disposable. So far, many different systems have been proposed. These systems typically do not fulfill all the requirements listed above and they are relatively expensive. An approach with a disposable part made of a plastic sheet was presented earlier.20 A set of wells was formed by hot embossing and the whole set was placed on top of the heaters. This system employs a relatively complicated microfabrication process and the disposable plate has to be customized. There is a need for a μPCR that is simple to manufacture, easy to operate, and economical enough to be disposable. The optional ability to be integrated into a complete μTAS system is highly desirable.
Here we report on a system (see Fig. 1), which fulfills all those demanding requirements. We propose to place a VRC14 on a microscope cover slip sitting on a micro-machined silicon structure integrated with both a thin film heater and a sensor. For the integrated sensors, the resistance temperature detector (RTD) type of sensor is a natural choice due to the simplicity of its fabrication, as there is only one metal deposition and two lithography steps required. Furthermore, the disposable cover slip part is not subject to any processing. The PCR system still maintains a high heating rate of greater than 30 K s−1 and a cooling rate of −20 K s−1.
 | ||
| Fig. 1 A photograph of a PCR chip soldered to a printed circuit board (PCB) with a square microscope glass cover slip. Four water-based samples, each with a volume of 1 μL, are placed on the glass and covered with 5 μL of mineral oil. The samples contain blue dye for easier visualization. Due to the soldering technique, we have achieved low resistance between the PCB and the chip as well as robust mechanical connection. Also, handling the whole PCB is much simpler than dealing with bare silicon chips. | ||
Theory and experimental
1. Theory
As described above, the ideal µPCR has to have good temperature control and uniformity across the reaction area. We propose to make a hollow reaction chamber integrated with both the heater and the sensor out of micromachined silicon, which is then connected by a narrow beam to the substrate. This beam serves as both a mechanical and a thermal connector. The PCR will be conducted on a glass microscope cover slip, thermally connected to the silicon by a thin film of oil (see the complete system in Fig. 1 and schematic diagram at Fig. 2, top). This configuration will keep the reaction chamber transparent, allowing optical excitation or detection from the bottom.21 The glass thermal conductivity coefficient λ is 1.1 W m−1 K−1, while the surrounding air has a thermal conductivity coefficient of only 0.025 W m−1 K−1, thus the temperature of the glass around the donut shape heater will be determined only by the temperature of the heater. The glass, which is the only part in contact with the PCR solution, is disposable. As it is not subject to any processing, it makes the PCR economical. | ||
| Fig. 2 Schematic cross-section of an individual heater (top) with a VRC, which is water-based droplet enclosed by oil, placed on the top of the heater. Both the heater as well as the sensor are made of a thin film of metal and thermally connected by placing them at the same silicon ring. Silicon is separated from the VRC by a thin sheet of glass, which transfers the heat from the heater to the sample. Schematic drawing of a second version of a thermal cycler (bottom). The lighter part is micromachined silicon with a thickness of 450 μm while the darker donut shapes represent the heater (outer) and sensor (inner). The temperature distribution is symmetrical along the axis of symmetry represented by the dashed line. Thermal conductance is given by the beam material, its length and cross section. Thermal capacitance is given by the double donut volume together with the volume of the VRC. | ||
Due to the high thermal conductivity of silicon compared to that of glass, the thermal cross talk between chambers is expected to be minimal. This would allow running of a number of PCR systems independently in close proximity to each other.
2. Finite element analysis
The proposed simple donut structure consisting of four heaters each connected by a beam to the substrate was modelled by the finite element analysis (FEA) software, ANSYS version 9.0.† As expected, the greatest thermal gradient was found to be along the beam axis. There was also a thermal gradient along the donut shape causing an unacceptably high temperature nonuniformity of 5 °C within the reaction area. It became obvious that the design had to be improved.The silicon device is symmetrical along the cantilever axis. We decided to take advantage of the symmetry and create a double donut shape (see Fig. 2) with the heater and sensor located at the inner donut. Both donuts are connected by two beams with thermal conductivities of at least half that of the inner donut. It results in temperature uniformity within the inner donut. The heat was dissipated almost entirely via the cantilevers, supporting our expectation that the cross talk between zones will be minimal. This design allows controlling the temperature of all four areas independently from each other, and thus we could run four different PCR protocols simultaneously.
The chip was designed with a bonding pad configuration identical to a standard leadless chip carrier (LCC-68) socket, so that it could be clamped into a conventional testing socket to determine device thermal parameters. Since our device thickness was only 0.45 mm as compared to the standard LCC chip, which has a thickness of about 3 mm, we added a plastic frame on the top of our chip to compensate for its smaller thickness.
3. Fabrication
The basic substrate for device fabrication was a conventional 4″ silicon wafer. We started off by depositing a 1 μm layer of silicon oxide by plasma enhanced chemical vapor deposition (PECVD). The SiO2 film serves as an electrical insulator between the silicon and the subsequent metal film. A 250 nm gold layer with a thin chrome adhesion layer was deposited by e-beam evaporation with a total sheet resistance of 0.11 Ω/□. We lithographically patterned the Au/Cr layer using 2 μm thick AZ7220 positive photoresist (Clariant Corp.) to form the heater, sensor, electrical lead outs, and contact pads. Both metals were etched by conventional etching solutions: gold by KI–I2 and chrome by (NH4)2Ce(NO3)6 based solutions. After etching of the metal sandwich, the photoresist was stripped by acetone, and the second lithography step was performed using 10 μm thick AZ4620 photoresist (Clariant Corp.). The thick photoresist was chosen to serve as a mask for silicon etching by deep reaction ion etching (DRIE) through the entire thickness of the silicon wafer. Besides preventing silicon from being etched by the DRIE, the photoresist also protected the gold lines. Silicon oxide was first etched by 7 ∶ 1 buffered oxide etch (BOE) solution to expose bare silicon and it was followed by DRIE (Bosch process).22 As the chip scribe lines were also patterned, the DRIE process produced individual chips and eliminated the need for dicing of the relatively fragile MEMS structures. The last process step was the individual chip cleaning by piranha solution (H2SO4–H2O2), rinsing by de-ionized (DI) water and drying by nitrogen flow.4. Device characterization
The electrical parameters of fabricated devices were determined by probing the device at the probe station (Cascade Microtech, Inc.) at different temperatures. The resistors' values were measured by a 4156C Semiconductor Parameter Analyzer (Agilent, Inc.).The resistance value R of a resistor versus temperature difference ΔT can be described by a simplified equation:
| R = R0(1 + αΔT) | (1) |
| Sensor resistance | 320 Ω |
| Heater resistance | 110 Ω |
| Sensor TCR | 0.33% K−1 |
| Sensor sheet resistance | 0.11Ω/□ |
| Unit heat conductance | 4.40 mW K−1 |
| Unit heat capacitance | 6.60 mJ K−1 |
| PCR thermal time constant | 1.74 s |
The thermal behavior of any system is described by the differential heat balance equation:
 | (2) |
Previously, a pulse method to derive thermal parameters of bolometers for infrared detection was published.23 In principle, bolometers exhibit a behavior similar to PCR devices, so we have used an identical testing method. The sensor under evaluation together with three external resistors formed a balanced Wheatstone bridge. It was powered by pulses with durations of 1 ms and a voltage amplitude of 5 V with a repetition rate of 1 pulse per second. A DC voltage signal with an amplitude between 0 and 1 V was superposed onto the pulses.23 The thermal capacitance H of the device was calculated from the derivative of temperature with respect to time. The temperature increase above ambient due to the applied DC voltage is a function of thermal conductance G. Obtained values of H and G were verified by direct measurement of the system’s time constant τ (equal to H/G). All measured and calculated electrical and thermal parameters are listed in Table 1.
5. Temperature distribution
In order to electrically and mechanically connect the chip, we soldered it to a PCB using a technique similar to flip-chip bonding. The solder formed the electrical and mechanical connection between the PCR device and the PCB (see Fig. 1).The PCR device was connected to the temperature control electronics as described later in this paper. The temperatures of the individual heaters were set approximately to 65, 85 and 94 °C and infrared (IR) images at wavelengths from 8 to 12 μm were captured. The camera’s temperature resolution was 0.1 K of the noise equivalent temperature difference (NETD) (see Fig. 3). As shown in Fig. 3, the temperature variation across the heaters is less than 1 °C, and thus the device is well-suited to perform the PCR sequence.
 | ||
| Fig. 3 An infrared image of a PCR device soldered to the PCB is shown here. Blue represents the cold zone and red represents the hot zone. The scale is shown on the right hand side. The bottom heater temperature was set to 95 °C, left and top to 85 °C and right heater temperature to 65 °C. All heaters are covered with a glass slip with a diameter of 12 mm. The temperature profile along the green line was extracted. The temperature variation within both zones of interest is within ±0.5 °C. It verifies the assumption as well as the FEA simulation results that the thermal non-uniformity within the zones matches the requirements for the PCR system. | ||
6. Control system
Temperature above ambient is controlled by modulating heating power. We have employed the pulse-width modulation (PWM) principle. It controls an average dissipated power by modulating the duty cycle of power pulses significantly shorter than the system’s time constant. The PWM is digital, and it is easy to implement with a LabView data acquisition card DAQ 6014-E (National Instuments, Inc.) controlled from a personal computer (PC). The value of the temperature sensor at the chip was used for a closed feed back mode. The proportional integrated derivative (PID) method was implemented to achieve fast heating. The maximum current supplied from the DAQ card is only 8.5 mA of current, which is not enough for heating the PCR chip to desired temperature. The card was then interfaced with the PCR chip by an integrated circuit IR 2121 (International Rectifiers, Inc.), a high speed MOSFET/IGBT driver. Its output is capable of supplying an electrical current as high as 1 A with a frequency of pulses of up to 10 kHz, capable of powering the PCR chip.The temperature sensor, together with two fixed and one adjustable resistor, formed a Wheatstone bridge. Its outputs were connected to an INA143US (Burr-Brown, Inc.) differential amplifier with a fixed gain of 10. Its output was linked with LabView software by the same card as the one which controlled the IR 2121. The complete PCB consists of four individual channels to run four PCRs in parallel.
The PCR device was calibrated to a temperature precision of better than 0.5 °C. The device calibration was performed in a temperature-controlled bath filled with Fluorinert™ 77 (3M Specialty Chemicals Division, Inc.). Its temperature was measured by temperature sensors TSic™ (IST-AG, Switzerland) calibrated with a precision of 0.1 °C in the range from 50 to 100 °C, soldered at the PCB next to the PCR device.
The output values from all four channels were stored in a LabVIEW setup file and used for the feedback measurement. The microscope glass cover slip was placed on the PCR chip. A VRC sample with a volume of 1 μL and 5 μL of oil was dispensed above the heaters (see Fig. 1). The above procedure precisely verified the temperature of the heater but not of the PCR sample itself, which could be different. The sample temperature was determined by melting curve analysis,24 which is described later in this paper. We found that the sample temperature is two degrees lower than the temperature of the heater at 94 °C and the setup file was corrected accordingly.
7. Thermal cycling
First, we ran the self-calibration procedure to optimize PID values of the controller in order to achieve a fast heating response, while the cooling rate was determined by the thermal time constant and surrounding temperature. From the thermal parameters listed in Table 1 it is expected that the device cooling time should be between one and two seconds as the temperature change from 94 to 55 °C is about 56% of the temperature difference between 94 °C and 25 °C. That would give the system a fast cooling rate between −20 to −40 K s−1, greatly exceeding the required minimal rate of −5 K s−1.8. Fluorescence detection
We used a mercury lamp with a Fluorescein (FITC) excitation/detection cube similar to the systems used earlier,25,26 with the fluorescence response detected by a pthoto multiplier tube (PMT) H5784-20 (Hamamatsu, K.K) with the gain set to about 5 × 104. The PCR chip was placed under an Axiotech Vario microscope (Zeiss, Inc.) mounted on an optical table. The whole measurement setup was covered with a black cloth in order to suppress the amount of ambient light entering the PMT in order to increase the optical detection limit. An oscilloscope measured and stored the value of the PMT signal amplitude.PCR device testing
1. Surface preparation
As described by Guttenberg et al,19 the glass surface for the VRC system has to be hydrophobic as well as oleophobic. We have tested a few different fluorinated silane solutions and preparation methods. The optimized coating consisted of cleaning the glass in a 3 ∶ 1 H2SO4–H2O2 mixture, followed by a DI water wash. The glass was then placed in YES-15E vacuum oven (Yield Engineering, Inc.) at room temperature together with a beaker containing 1 mL of a fluorosilane [(heptadecafluoro-1,1,2,2-tetrahydrodecyl)trimethoxysilane] (Gelest, Inc.). The oven was then evacuated to reach a residual pressure of below 1 Torr and the oven temperature was raised to 150 °C while pumping was continued. Silane was vaporized and reacted with the glass surface. After 2 to 5 h, the pumping was stopped, the oven was vented with nitrogen and glass slides were removed from the oven. Results of surface treatment were verified by the contact angle method using contact angle system Model OCA 30 (Dataphysics, GmbH). The contact angle of a water droplet was 110 degrees while a M5904 mineral oil (Sigma Inc.) droplet had a contact angle of 70 degrees.2. Sample preparation
As a test vehicle, we have used 940 template copies of a 208 base pair fragment (Maxim Biotech, Inc.) of the gene encoding for human glycerinaldehyde 3-phosphate dehydrogenase (GAPDH). 5′-CTCATTTCCTGGTATGACAACGA-3′ was used as a forward primer and 5′-GTCTACATGGCAACTGTGAGGAG-3′ (Research Biolabs, Inc.) as a reverse primer. The PCR mixture was prepared in a 50 μl stock solution as recommended by the manufacturer (Qiagen, Inc.) with two exceptions: SYBR Green (Invitrogen, Inc.) was diluted to a final concentration of 1 ∶ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and bovine serum albumin (Carl Roth, Inc.) was added in a final concentration of 1%.
000 and bovine serum albumin (Carl Roth, Inc.) was added in a final concentration of 1%.
3. Real-time PCR results
The PCR stock solution prepared as described in previous section was divided into two parts, where 1 μl was used for the PCR chip and the remainder was used in a conventional thermocycler (MJ Research, Inc.) as a reference. For both experiments, the PCR mixture was covered by 5 μL of mineral oil (Sigma, Inc.). Thermocycling conditions were as follows: 5 min at 94 °C (initial denaturation), followed by 50 cycles of 1 min at 94 °C (denaturation), 1 min at 58 °C (annealing) and 1 min at 72 °C (extension) with a final step of 10 min at 72 °C. One minute per thermal step of the PCR cycle is longer than normal.3 However, it assures that the system reaches thermal equilibrium and the enzymatic reaction is completed during each step. This is more important at this point of time than the optimization of each step to make the PCR system fast.We are currently developing a fast PCR system capable of 40-cycle process in 5 min or less,27 which is not subject of this paper.
For melting curve analysis24 the sample was cooled down to 65 °C for 1 min after which the temperature was continuously raised to 95 °C with a heating rate of 0.01 °C s−1. During the operation, both the fluorescence signal as well as the temperature sensor value was recorded simultaneously.
The next step was the calculation of an average value of the fluorescence signal during the end of the extension phase at 72 °C. In order to extract the fluorescence output signal from the PCR cycles, a short program was prepared using Fortran. The program input parameters were the center of the first data block shown by a blue arrow in Fig. 4, the length of the data interval and the number of intervals. The program then averaged the signal from the interval and associated it with a cycle number for all 50 cycles. The same procedure was repeated for the fluorescence signal at 94 °C (indicated by the red arrow in Fig. 4) in order to obtain a baseline signal to be subtracted from the PCR output signal at 72 °C. The subtracted data set was approximated with a sigmoidal function:
 | (3) |
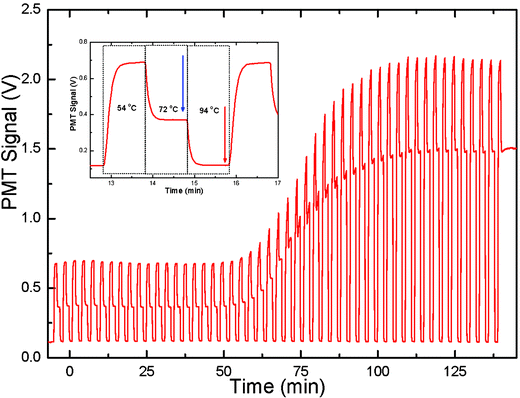 | ||
| Fig. 4 Fluorescence signal detected from a PCR chip versus time over 50 cycles. The fluorescence signal from a single PCR cycle is shown in the inset. The real time PCR data points were obtained by subtracting the fluorescence signal at a temperature of 94 °C (shown by a red arrow in the inset) from the signal at a temperature of 72 °C (shown by a blue arrow in the inset). The cycle threshold (CT) value of this PCR sample was about 20. | ||
The PCR protocol was run with different concentrations of template copies varied from 10 up to one million. Calculated parameters of x0 were plotted versus the number of templates showing the PCR standard curve.
As mentioned above, after thermal cycling of the PCR device a melting curve analysis28–30 was performed in order to determine the purity of the PCR. The fluorescence signal was approximated by a modified sigmoidal function:
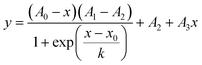 | (4) |
The fitting error shows only a small difference between the measured data and the fitting curve, showing that there is only one PCR product with a limited amount of by-products. The purity of products was proven by the results of capillary electrophoresis (see inset in Fig. 5).
 | ||
| Fig. 5 Fluorescence signal from melting curve analysis (black line hidden behind the red line) approximated by a sigmoidal function shown in eqn (4) (red line). The deviation between measured data and the sigmoidal function is negligible demonstrating the purity of the PCR product. The negative value of its derivation is shown in blue. Results of capillary electrophoresis (see the inset) demonstrate the PCR product purity. | ||
Conclusions
We have demonstrated a high performance PCR system where the disposable portion is a microscope cover slip. The non-disposable part is a simple micromachined silicon chip directly soldered to a PCB for mechanical and electrical connections as well as for easy handling. A sample for the PCR with a 1 μL volume was encapsulated by oil thus forming a VRC.The most important contribution of this work is the development of a system, which enables the micromachined chip to be separated from the disposable glass slide where the PCR takes place. The disposable part of the PCR system completely eliminates the danger of sample to sample cross contamination. As the glass does not require any processing except for the cleaning and fluorosilane coating, it costs just a few pennies. The thermal properties of the system are dominated by the sample volume, which can be further reduced making the PCR even more economical.
The whole system, as described above, can be mass-produced using well-established micromachining processes. We are currently developing a battery operated system: the PC will be replaced by a single chip processor, the fluorescence microscope and PMT detector will be substituted by a blue light emitting diode (LED), a miniaturized optical unit containing a photodiode and the optical signal processing will be integrated at the PCB with the microchip controller. Our goal is to complete the development of a truly economical and portable lab-on-a-chip system.
Acknowledgements
We would like to thank our co-worker Sun Wanxin for the quick development of the Fortran program to extract data from the fluorescence signal, Sharon Oh for her help with the manuscript preparation, Vitek Zahlava (Czech Technical University) for the PCB design and assembly and Petr Taras from IST-AG (Watwill, Switzerland) for his help with TSic™ temperature sensors and their calibration. The authors are also grateful to the Institute of Bioengineering and Nanotechnology, Singapore as well as the Agency for Science, Technology and Research, Singapore for their financial support.We would also like to thank the two anonymous reviewers who by their comments helped us to improve this paper.
References
- R. K. Saiki, S. Scharf, F. Faloona, K. B. Mullis, G. T. Horn, H. A. Erlich and N. Arnheim, Science, 1985, 230, 1350–1354 CrossRef CAS.
- M. Hashimoto, P.-C. Chen, M. W. Mitchell, D. E. Nikitopoulos, S. A. Soper and M. C. Murphy, Lab Chip, 2004, 4, 638–645 RSC.
- K. B. Mullis, F. Feree and R. A. Gibbs, Polymerase Chain Reaction, 1994, Binkhauser, Boston Search PubMed.
- P. Wilding, M. A. Shoffner and L. J. Kricka, Clin. Chem., 1994, 40, 1815–1817 CAS.
- J. B. Findlay, S. M. Atwood, L. Bergmeyer, J. Chemelli, K. Christy, T. Cummins, W. Donish, T. Ekeze, J. Falvo, D. Patterson, J. Puskas, J. Quenin, J. Shah, D. Sharkey, J. W. H. Sutherland, R. Sutton, H. Warren and J. Wellman, Clin. Chem., 1993, 39(9), 1927–1933 CAS.
- K. E. Petersen, Proc. IEEE, 1982, 70, 420–457 CrossRef CAS.
- A. C. R. Grayson, R. S. Shawgo, A. M. Johnson, N. T. Flynn, Y. Li, M. J. Cima and R. Langer, Proc. IEEE, 2004, 92, 6–21 CrossRef CAS.
- M. G. Roper, C. J. Easley and J. P. Landers, Anal. Chem., 2005, 77, 3887–3894 CrossRef CAS.
- E. Oosterbroek and A. van den Berg, Lab-on-a-Chip, Miniaturized Systems for (Bio) Chemical Analysis and Synthesis, 2005, Elsevier, Netherland Search PubMed.
- M. A. Northrup, B. Bennet, D. Hadley, P. Landre, S. Lehew, J. Richards and P. Stratton, Anal. Chem., 1998, 70, 918–922 CrossRef CAS.
- M. U. Kopp, A. J. de Mello and A. Manz, Science, 1998, 280, 1046–1048 CrossRef CAS.
- J. Liu, M. Enzelberger and S. Quake, Electrophoresis, 2002, 23, 1531–1536 CrossRef CAS.
- A. F. R. Huhmer and J. P. Landers, Anal. Chem., 2000, 72, 5507–5512 CrossRef CAS.
- I. Schneegass, R. Braeutigam and H. M. Koehler, Lab Chip, 2001, 1, 42–49 RSC.
- J. Cheng, M. A. Shoffner, K. R. Mitchelson, L. J. Kricka and P. Wilding, J. Chromatogr., A, 1996, 732, 151–158 CrossRef CAS.
- J. N. Yang, Y. J. Liu, C. B. Rauch, R. L. Stevens, R. H. Liu, R. Lenigk and P. Grodzinski, Lab Chip, 2002, 2, 179–187 RSC.
- Y. S. Shin, K. Cho, H. L. Sun, S. Chung, S.-J. Park, C. Chung, D.-C. Han and J. K. Chang, J. Micromech. Microeng., 2003, 13, 768–774 CrossRef CAS.
- K. Shen, X. Chen, M. Guo and J. Cheng, Sens. Actuators, B, 2005, 105, 251–258 CrossRef.
- Z. Guttenberg, H. Mueller, H. Habermueller, A. Geisbauer, J. Pipper, J. Felbel, M. Kielpinski, J. Scriba and A. Wixforth, Lab Chip, 2005, 5, 308–317 RSC.
- Q. Zou and U. Sridhar, Miniaturized multi-chamber thermal cycler for independent thermal multiplexing, US pat. 6509186, 2004 Search PubMed.
- K. Sun, A. Yamaguchi, Y. Ishida, S. Matsuo and H. Misawa, Sens. Actuators, B, 2002, 84, 283–289 CrossRef.
- F. Laermer and A. Schilp, Method for anisotropic plasma etching of substrates, US pat. 5498312, 1996 Search PubMed.
- P. Neuzil and T. Mei, Appl. Phys. Lett., 2002, 80, 1838–1840 CrossRef CAS.
- R. G. Rutledge, Nucleic Acids Res., 2004, 32, e178 CrossRef CAS.
- P. K. Dasgupta, I. Eom, K. J. Moris and J. Li, Anal. Chim. Acta, 2003, 500, 337–364 CrossRef CAS.
- N. C. Cady, S. Stellick, M. V. Kunnavakkam and C. A. Batt, Sens. Actuators, B, 2005, 107, 332–341 CrossRef.
- P. Neuzil, C. Zhang, J. Pipper, S. Oh and Z. Lang, Nucleic Acids Res., submitted for publication Search PubMed.
- M. Fixman and J. J. Freire, Biopolymers, 1977, 16, 2693–2704 CrossRef CAS.
- S. Wilkening and A. Bader, J. Biomol. Tech., 2004, 15, 107–111 Search PubMed.
- E. Lyon, A. Millison, M. C. Lowery, R. Woods and C. T. Wittwer, Clin. Chem., 2001, 47, 844–850 CAS.
Footnote |
| † Elements SHELL-57 with real constants of 450 µm for silicon and 170 µm for glass were used. |
| This journal is © The Royal Society of Chemistry 2006 |
