Maskless microfabrication of thermosensitive gels using a microscope and application to a controlled release microchip†
Ryo
Yoshida
*,
Kazuya
Omata
,
Kotaro
Yamaura
,
Masaya
Ebata
,
Masahiro
Tanaka
and
Madoka
Takai
Department of Materials Engineering, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: ryo@cross.t.u-tokyo.ac.jp
First published on 9th August 2006
Abstract
Here we show a simple and convenient method to prepare micropatterned gels by the use of a microscope, without large-scale or special-order experimental setup. UV light focused by an objective lens was locally irradiated to a pre-gel solution in a microchannel. This method would be useful for preparing microgels at target positions in microchips. A controlled drug-release microchip has actually been fabricated by utilizing this local photo-irradiation method, and pulsatile drug release in response to temperature changes was demonstrated.
Introduction
Recently, stimuli-responsive polymer gels have attracted attention as functional soft materials in the research field of microfluidic systems. Several applications, such as a smart microvalve or micropump to switch the direction of flow automatically or extrude fluid have been attempted.1,2 For microfabrication or micropatterning of gels, lithography methods using a photomask have typically been employed.3,4 Recently, micropatterning by a maskless projection method, using an LCD projector has become popular for surface modification of polymers.5 In the case of photolithography, an additional process to make a photomask is needed. As for maskless projection methods, in addition to the fact that special-order setup is necessary, the fineness of the micropatterned gels depends on the resolution of the projector.Here we show a simple and convenient method to prepare micropatterned gels by use of a microscope, without large-scale or special-order experimental setup. UV light focused by an objective lens was locally irradiated on to a pre-gel solution in a microchannel. By moving the sample stage, a microgel with any shape can be prepared at any position in the microchannel. This method would be useful for preparing microgels at target positions in microchips, for example as a microvalve or micropump. In this study, a controlled drug-release microchip has actually been fabricated by utilizing this local photo-irradiation method, and pulsatile drug release in response to temperature changes was demonstrated.
Experimental
Preparation of microgel
At first, using N-isopropylacrylamide (NIPAAm), acrylic acid (AAc, 3 mol%), and 2,2′-azobis(isobutyronitrile) (AIBN) as an initiator, poly(NIPAAm-co-AAc) was synthesized by radical polymerization in methanol. Then the carboxyl groups of the polymer were substituted by azidophenyl groups by the reaction with 4-azidoaniline hydrochloride and 1-(3-dimethyl-aminopropyl)-3-ethylcarbodiimide hydrochloride.After dissolving the polymer into methanol, the polymer solution was injected into a speculum plate with a spacer of 70 µm thickness. The plate was set up on a motor-driven x-y sample stage, which was equipped with a fluorescence microscope (DIAPHOT, Nikon). UV light from an Hg lamp (100 W, 300 cd, peak wavelength: 365 nm) was condensed by an objective lens (Plan 20, Nikon) to focus on the stage, and the spotlight was irradiated to the plate. By moving the stage continuously and sweeping the spotlight, a linear microgel was prepared.
For preparation of microgels by photo-polymerization, NIPAAm, AAc, methylenebisacrylamide (MBAAm), and 2,2-dimethoxy-2-phenylacetophenone as a photo-initiator, were dissolved in methanol. The pre-gel solution was injected into the speculum plate, and the UV spotlight, condensed by the objective lens (Plan 10, Nikon), was irradiated for 15 s.
Fabrication of a microchip
A drug-release microchip composed of three PDMS layers was fabricated (see Fig. 4). Each layer was fabricated by a typical soft lithography method, and then they were bonded to each other, after O2 plasma treatment. The width and the height of the microchannel were 500 µm and 200 µm, respectively.The pre-gel solution for photo-polymerization was injected into the microchannel of the microchip. The UV spotlight condensed by the objective lens (Plan 10) was locally irradiated to the region around the pillar for 15 s. After gelation, the gel was washed and deswelled by drying. The colored solution of Ru(bpy)3Cl2, as a model drug, was loaded in the drug reservoir by injecting into the microchannel. Pure water was supplied to both the drug-flow and water-flow channel by using an HPLC pump with a flow rate of 0.1 ml min−1. The effluent from the drug flow channel was introduced to a single path UV detector (UV-2075, JASCO) and the release rate of the model drug was continuously monitored from the absorbance at 420 nm wavelength in response to stepwise temperature changes.
Results and discussion
In order to prepare the microgel by photo-crosslinking, poly(NIPAAm) (PNIPAAm) with photo-reactive azidophenyl groups was used. By irradiating the UV spotlight locally to the polymer solution and sweeping the spotlight, a linear microgel can be obtained. Fig. 1(a) shows the effect of the sweeping rate on the line width of the gel at swollen (20 °C) and unswollen states (60 °C). The swelling ratio, defined as a ratio of the two widths, is also shown. As the sweeping rate increases, the width decreases in both the swollen and unswollen states. However, the swelling ratio increases with the increase in sweeping rate. When the sweeping rate is high, the gelation area becomes narrow because the local exposure time of UV light becomes short. And also, due to the short UV exposure time, crosslinking density becomes small. As a result, the swelling ratio of the prepared gel increases. | ||
| Fig. 1 (a) Line width of the linear PNIPAAm gel (△: 20 °C and ▲: 60 °C) prepared by the photo-crosslinking method and the swelling ratio (○) as a function of sweeping rate of the UV spotlight. (b) Swelling behavior of the linear gel prepared by changing the sweeping rate stepwise between 1 and 10 µm s−1 (upper: 60 °C, lower: 20 °C, scale bars: 100 µm). | ||
Fig. 1(b) shows the prepared linear gel when the sweeping rate was changed stepwise between 1 and 10 µm s−1 during the irradiation. A linear gel with a narrower part in the center was obtained. When the gel swells with decreasing temperature, it was observed that the narrower part deformed, exhibiting a wavy shape. As mentioned above, the swelling ratio of the narrow part becomes higher than outer thicker parts. Consequently, the narrower part is under pressure when it swells in the direction of length. This constraining force leads to deformation in the perpendicular direction to the length, and thus a wavy shape is observed. By this microfiguring method, several shapes of micrometre-sized gels can be easily prepared. Fig. 2 shows the examples of characters and figures made of PNIPAAm gels. In the swollen state, the gels exhibit wavy deformation as observed in Fig. 1(b). The wavy shape in the swollen state returns to the original shape when the gel shrinks.
 | ||
| Fig. 2 PNIPAAm microgel characters (left) and figures (right) prepared by photo-crosslinking (upper: 60 °C, lower: 20 °C, scale bars: 500 µm). | ||
In this local photo-irradiation method, an array of microgels can be prepared easily by irradiating the UV spotlight without sweeping. In addition to the photo-crosslinking method, we tried to prepare a thermosensitive microgel array by photo-polymerization. Fig. 3A shows the fabrication process of a microgel array by repeated on-off switching of UV irradiation and moving the sample stage. The UV spotlight focused by an objective lens was irradiated locally to the monomer solution including a photo-initiator. After 15 s, the spotlight was turned off. Then it was observed that the microgel with a diameter of about 100 µm was formed within the region where the spotlight was irradiated. After that, the sample stage was moved and the spotlight was irradiated again to prepare another microgel next to the first one. By repeating this process, microgels which stand in a line can be fabricated. Generally, by using an objective lens with higher magnification, smaller microgels can be prepared. And also, as the concentration of monomer, crosslinker and initiator decreases, the size of the prepared microgel decreases, although the gelation time becomes longer. So far, it has been found that the diameter of the microgel could be reduced to about 20 µm by this photo-polymerization method.
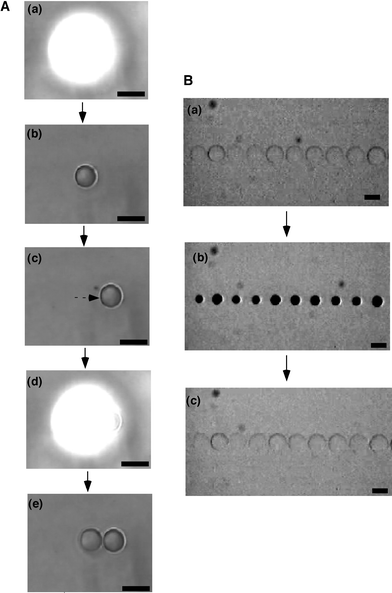 | ||
| Fig. 3 Preparation of PNIPAAm microgel array by local photo-irradiation (scale bars: 100 µm). A: (a) 1st irradiation of UV spotlight (15 s), (b) formation of 1st microgel, (c) movement of stage, (d) 2nd UV irradiation (15 s), (e) formation of 2nd microgel. B: Swelling–deswelling changes of the PNIPAAm microgel array in response to stepwise temperature changes between 20 °C (a,c) and 40 °C (b). | ||
Fig. 3B shows the PNIPAAm microgel array obtained by this method. At lower temperature, the microgels are in the swollen state (diameter, d = 120 µm), and there is no gap between the gels. With increasing temperature, the gels deswell (d = 60 µm) and make a gap between them. By decreasing the temperature, the gels swell again and the gap is closed. The response time for swelling and deswelling was about 3 minutes. Such gap-forming changes of the gels might be useful as a microvalve to open and shut the flow automatically in microfluidic systems.
As an example of one of the applications of these maskless local photo-irradiation methods to a micro-device, a controlled drug-release microchip was fabricated (Fig. 4). The top layer has a water-flow channel and the space for fixing the microgel. In the space, there is a small pillar to fix the microgel. The middle layer has a diaphragm (thickness: 50 µm) to open and close the drug reservoir by swelling and deswelling of the gel. By employing the diaphragm structure, we can avoid a direct contact of the microgel with the drug solution. The bottom layer has the drug reservoir and drug-flow channel.
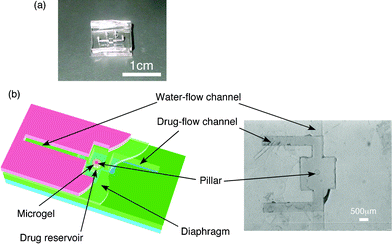 | ||
| Fig. 4 Drug-release microchip: (a) general photograph and (b) schematic drawing (left) and the top view around drug reservoir (right). | ||
A thermosensitive microgel was prepared in the microchannel by local UV irradiation. At 20 °C, the gel swelled to push the diaphragm and close the drug reservoir (Fig. 5B(a)). When temperature increased to 50 °C, the gel deswelled to open the drug reservoir. Since the gel became opaque, the drug reservoir could not be observed directly (b). When the temperature decreased to 20 °C and the gel swelled again, the drug reservoir was already vacant (c). This means that the model drug was completely released while the microgel deswelled. Fig. 5C shows the release profile of the model drug from the microchip in response to the temperature changes. It was actually observed that the drug was released after increasing the temperature. So far there have been several studies into on-off regulation of drug-release by using thermosensitive gels.6 This type of microchip would be applicable as an intelligent and disposable drug delivery patch to release an antipyretic only when the body temperature increases.
 | ||
| Fig. 5 Drug-release behaviours from the microchip in response to stepwise temperature changes. A. Cross-sectional illustration. B. Photograph of top view; (a) 20 °C, (b) 50 °C, (c) 20 °C. C. Pulsatile drug-release pattern after stepwise temperature increase from 20 °C to 50 °C. | ||
For microfabrication of gels, the local photo-irradiation methods utilizing a microscope presented here would be useful as an easy and simple method which does not need another process for making a photomask or special-order experimental setup. In particular, this maskless method will be effective for preparing microgels in microchannels because troublesome processes for alignment of a photomask to the channel are not needed. Further, several kinds of gels could be placed in the same microchannel by repeating irradiation and replacement of another monomer solution. Since any shape of gel can be created by this method, application as a new manufacturing method for soft microactuators, microgel valves, gel displays, etc. is expected.
References
- D. T. Eddington and D. J. Beebe, Adv. Drug Delivery Rev., 2004, 65, 199–210 CrossRef.
- B. Ziaie, A. Baldi, M. Lei, Y. Gu and R. A. Siegel, Adv. Drug Delivery Rev., 2004, 56, 145–172 CrossRef CAS.
- G. Chen, Y. Imanishi and Y. Ito, Macromolecules, 1998, 31, 4379–4381 CrossRef CAS.
- Z. Hu, Y. Chen, C. Wang, Y. Zheng and Y. Li, Nature, 1998, 393, 149–152 CrossRef CAS.
- J. Kobayashi, M. Yamato, K. Itoga, A. Kikuchi and T. Okano, Adv. Mater., 2004, 16, 1997–2001 CrossRef CAS.
- R. Yoshida, K. Sakai, T. Okano and Y. Sakurai, Adv. Drug Delivery Rev., 1993, 11, 85–108 CrossRef CAS.
Footnote |
| † The HTML version of this article has been enhanced with colour images. |
| This journal is © The Royal Society of Chemistry 2006 |
