Rapid heterogeneous liver-cell on-chip patterning via the enhanced field-induced dielectrophoresis trap
Chen-Ta Ho†a, Ruei-Zeng Lin†b, Wen-Yu Changa, Hwan-You Changbc and Cheng-Hsien Liu*ac
aDepartment of Power Mechanical Engineering, National Tsing Hua University, Hsinchu, Taiwan 300, ROC. E-mail: liuch@pme.nthu.edu.tw; Tel: +886-3-5715131 ext: 33706
bInstitute of Molecular Medicine, National Tsing Hua University, Hsinchu, Taiwan 300, ROC
cMicroelectromechanical Systems Institute, National Tsing Hua University, Hsinchu, Taiwan 300, ROC
First published on 3rd May 2006
Abstract
Biomimetic heterogeneous patterning of hepatic and endothelial cells, which start from randomly distributed cells inside the microfluidic chamber, via the chip design of enhanced field-induced dielectrophoresis (DEP) trap is demonstrated and reported in this paper. The concentric-stellate-tip electrode array design in this chip generates radial-pattern electric fields for the DEP manipulation of the live liver cells. By constructing the geometric shape and the distribution of stellate tips, the DEP electrodes enhance the desired spatial electric-field gradients to guide and snare individual cells to form the desired biomimetic pattern. With this proposed microfluidic chip design, the original randomly distributed hepatocytes inside the microfluidic chamber can be manipulated in parallel and align into the desired pearl-chain array pattern. This radial pattern mimics the lobular morphology of real liver tissue. The endothelial cells, then, are snared into the additional pearl-chain array and settle at the space in-between the previous hepatic pearl-chain array. By this cell-lab chip, we demonstrate the in vitro reconstruction of the heterogeneous lobule-mimetic radial pattern with good cell viability after cell patterning. This work reports the rapid in-parallel patterning of the dual types of live liver cells via the enhanced DEP trap inside the microfluidic chip.
Introduction
A variety of recent progresses in tissue engineering have been dedicated to the developments of cell-based artificial tissues.1–4 Cellular patterning techniques, which provide the basis development for rebuilding cell blocks, play a crucial role in a series of applications such as tissue engineering,5–7 cell-based biosensors,8,9 medical diagnostics,10 and drug delivery.9,11 In the traditional tissue engineering, porous scaffolds have been used to provide with physical and chemical cues for cellular differentiation and assembly into tissue.3,4 Although advanced biodegradable scaffolds,12,13 which morphologically mimic the human tissue, are developed as the cultured matrix for the cell attachment, it is still insufficient to guide, place and distribute the heterogeneous cells to reconstruct complicated architectures of tissue especially like kidney3 and liver.4 In particular, hepatic sinusoids, the special liver's micro-vascular systems which are lined by liver sinusoid endothelial cells to form a radial pattern, are essential for normal liver functions and hepatocyte survival.14 Thus, fine cell-patterning techniques are important in tissue engineering because the adequate positioning of both hepatic and endothelial cells to reconstruct the complex liver tissue according to its native architecture is the major challenge of liver tissue engineering.5,6In recent years, some strategies have been focusing on chemically modifying the substrate with cell-adhesion protein by photolithography15,16 to increase cell adhesion as well as approach fine cell-patterning resolution. Anyway, the chemicals used in micropatterning processes mostly are toxic to cells and influence the final cell viability. Instead, the microcontact printing technique17,18 provides good resolution and a flexibility method for cell-patterning but it still lacks the capability to pattern cells with more than two types of cells due to the limited self-assembly monolayer ligands. Furthermore, microfluidic patterning using microchannels19 and laminar flow patterning20 has recently been demonstrated with the ability capable of patterning multiple cells via selectively delivering the materials for cell adhesion to the desired area of the substrate. However, the microfluidic patterning technique with coarse cell-patterning resolution is insufficient for patterning a complex structure like liver tissue. The laser-guided direct writing21 is another technique adopted to generate precise cell patterns. However, some drawbacks do arise, not only the inability to control multiple cells simultaneously but also for the concern of viability due to the high power energy of laser. Thus, the development of high-resolution cell pattering, which is also capable of rapidly controlling multiple types of cells with good cell viability to reserve cell–cell interactions as well as potentially modulating cell behaviour, is important and challenging.
Compared with the passive cell-patterning techniques addressed above, an active manipulation method, that of dielectrophoresis (DEP),22,23 has been recently widely used and demonstrated with the functions of trapping,24,25 separation,26–28 fractionation,29 sorting,30,31 and handling32 for beads and a few specific bioparticles. For rapid manipulation and patterning on delicate cells like liver cells, earlier efforts have been demonstrated by taking advantage of negative DEP force for cell patterning33 and position.34,35 Using negative DEP as the cell-patterning force, which repulses the cells to the region of local electric-field minimum, could reduce the extra energy acting on the cells, but the cell aggregation in groups observed is a drawback, which is unfavourable in clearly forming fine patterns. On the contrary, the positive DEP force, which attracts cells to the region of local electric-field maximum, can provide interesting functions such as cell positioning and has been applied to the applications of cell registration,36 living cell arrays37 and microbial biofilms.38 Although the positive DEP technique possesses the ability potentially capable of manipulating thousands of cells in parallel with the single-cell resolution, few of these technique developments were demonstrated to reconstruct complex patterns of heterogeneous cells for human tissue yet.
The appropriate fine pattern of heterogeneous cells, such as the classic lobule consisting of hepatic and endothelial cells [Fig. 1(a)], is a challenge to researchers and an important step in tissue engineering toward the development of artificial liver. For this goal, we report here the design, microfabrication, and characterization of a rapid liver-cell patterning microfluidic chip, which utilizes the enhanced positive DEP to build the pattern of heterogeneous liver cells mimicking the lobular morphology of real liver tissue in vivo. A specific snaring electrode array design for the DEP manipulation of the liver cells, to construct the radial pearl-chain patterns of liver cells, is reported in this paper. The microfluidics combined with the enhanced spatially radial electric-field patterns in our chip provide a scale-match tool capable of manipulating, snaring, and patterning a mass of individual cells in parallel.
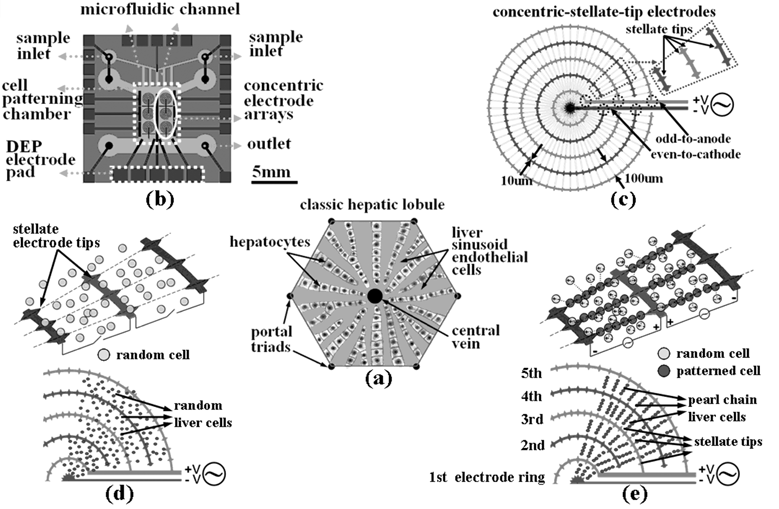 | ||
| Fig. 1 The illustration, chip design and principles for the liver-cell patterning microfluidic chip. (a) The illustrated configuration for one unit of the classic hepatic lobule. (b) Top schematic view of the cell-patterning microfluidic chip. This chip is composed of a transparent glass substrate embedded with the matrices of concentric-stellate-tip array electrodes and a PDMS top cover. (c) The enlarged view for a unit of concentric-stellate-tip array electrodes. (d) Liver cells are spatially randomly distributed before the ac DEP voltage is applied. (e) The energized concentric-stellate-tip array electrodes provide well-defined local electric-field maxima. Liver cells are snared and aligned along the field-induced orientation to form the radial pearl-chain two-dimensional patterns due to the positive DEP after the ac DEP voltage is applied. | ||
Principles, design and simulation
Field-induced dielectrophoresis
Dielectrophoresis (DEP) is a phenomenon caused by the induced dipole of the polarizable particles in the solution under non-uniform electric fields. This phenomenon has been applied to the manipulation of bioparticles such as a virus, bacteria, neurons, and few specific types of cells in microfluidics.24–29 The time-averaged DEP force,22,23FDEP, acting on a spherical particle of radius r suspended in a medium of relative permittivity εm is given by| FDEP=2πr3εm Re[fCM(ω)]∇E2rms | (1) |
 | (2) |
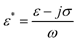 | (3) |
| FDEP ∝ ∇E2rms | (4) |
Through the specific design of the electrode geometry and distribution, the desired electric-field morphology could be formed to manipulate target particles/cells to achieve the desired particle/cell pattern with either positive or negative DEP based on appropriate ac potential frequency and medium conductivity. The concept that the control of the electric-field gradient is equivalent to the control of the cell pattern in our cell-patterning bio-chip is what we would like to highlight here. In this work, we take advantage of the concept of controlling the gradient of electric field square to design the concentric-stellate-tip array electrodes for snaring cells to form the desired pattern based on positive DEP in microfluidics. The concentric-stellate-tip array electrodes provide inhomogeneous electric-field gradients to manipulate cells via field-induced DEP. By constructing the geometric shape and the distribution of stellate-tips electrodes, the DEP electrodes could generate the well-defined spatially radial patterns of electric-field gradients to guide individual cells in microfluidics from random distribution onto the substrate spatially in order.
The chip design for liver-cell patterning
The liver is considered as a difficult object to be reconstructed by tissue engineering because of its complex cellular architecture. The liver is morphologically divided into lobules which take the shape of irregular polygonal prisms as illustrated in Fig. 1(a). From the cross-section view, the lobule is filled with cords of liver parenchyma cells, hepatocytes, which radiate from the central vein and are separated by sinusoid-like vascular endothelial lining cells. In a three-dimensional morphology, the liver tissue is more like regularly branching and interconnecting sheets which extend in parallel to the long axis of the lobule and radiate out from the center. This architecture enlarges total contact area in the organ level and enables rapid mass transfer from the sinusoid blood flow into the liver parenchyma for detoxification or metabolic functions. From the view at the cellular level, the direct cell-to-cell contact between heterogeneous cells, in particular spatial orientation, is also essential for normal development and organogenesis. For the above reasons, the appropriate patterning of liver and endothelial cells to establish a basic unit of liver tissue, the lobule, for cell-to-cell interaction is the most fundamental and key step to successful liver tissue engineering.3,4To approach the heterogeneous-cells patterning of complex liver tissue, we take advantage of the DEP force to construct the cell pattern of liver tissue. The schematic diagram, chip design, and principles for our cell-patterning microfluidic chip are illustrated as shown in Fig. 1. The cell-patterning microfluidic chip is composed of planar electrodes fabricated on a transparent glass substrate and a PDMS top cover [Fig. 1(b)]. The concentric electrode arrays are micromachined above the glass substrate for the DEP manipulation of cells. The sample inlets/outlets, microfluidic channels and cell-patterning chamber are micromolded by using the transparent PDMS. We mimic the morphology of a classic lobule, the basic unit of liver tissue, to design the DEP snaring electrodes in our chip for the cell patterning in vitro. To establish the radial pattern of liver lobule, the concentric-ring array electrodes are designed to generate the radial electric fields. Fig. 1(c) depicts the enlarged view of a unit of concentric-stellate-tip array electrodes, which has the specific stellate tips for the purpose of electric-field enhancement to mimic the lobular morphology of real liver tissue. Each ring electrode in the multiple concentric-ring array electrodes, which are designed to generate radial electric field for the DEP manipulation of cells, has the width dimension of 10 µm. The odd-order ring electrodes and the odd-order ring electrodes are electrically connected by themselves, respectively. The stellate tips repeatedly appear along the 1st ring electrode and the 2nd ring electrode every π/16 radian angle, and appear along the 3rd, 4th and 5th ring electrode every π/32 radian angle for the purpose of achieving the denser cell pattern around the outer rings. All the stellate tip electrodes are homocentric in architecture and the shape of a concentric-stellate tip is an isosceles triangle with an angle of 60° and the height of 10 µm. The gap between opposite tips at the adjacent ring electrodes is 100 µm, which is designed for snaring about 8 cells in a “pearl-chain string”. The odd-order rings of the concentric-ring array electrodes start from the 0° angle with a connected common electrode, rotate counter-clockwise, and end at the angle of 355°. The even-order rings of the concentric-ring array electrodes start from the angle of 360° with the other common electrode connected, rotate clockwise, and end at the angle of 5°. The design of concentric-stellate-tip array electrodes proposed in this paper could easily be increased the ring number to cover the cell-patterning area of centimetre in diameter or even the larger scale to enlarge the area and amount of patterned cells.
In addition, the design of the concentric-ring array electrodes could provide the formation of radial electric field to manipulate randomly distributed cells into the radial pearl-chain patterns via DEP force. With the pure ring electrodes (without the stellate tips proposed in this paper), the distribution of pearl-chain cells above the area of the concentric-ring array electrodes (cell-patterning region) would be randomly displayed. The position probability of DEP-snared cells is dominated by the random distribution of the radial electric field. However, the distribution of the radial pearl-chain cells could be highly improved by regularly adding the stellate tips on the concentric-ring array electrodes. This effect comes from the enhancement of the electric field because the concentric-stellate-tip array electrodes could not only enhance but also provide local maximum gradients of electric field with the precise position inside the concentric-ring array to snare cells via DEP force. The concentric-ring array electrodes provide a global radial electric field for the initial formation of cell patterning at the first stage. In the meantime, the concentric stellate-tips, which act as the local destination directors, provide the local maxima of electric-field gradients to more precisely snare the cells to form the desired cell pattern. The effect of concentric-stellate-tip array electrodes could refine the cell position to result in precisely positioning the radial pearl-chain cell patterns. Through the design of the concentric-stellate-tip array electrodes, the field-induced DEP could rapidly snare numerous cells in parallel to create more appropriate cell pattern of radial pearl-chains.
The operation principle of cell-patterning chip
Fig. 1(d) and (e) illustrate the operation principle of our liver-cell patterning chip. After the liver cells are introduced into the microfluidic chamber with continuous flow input, the cells are guided along the stream and randomly distributed, as illustrated in Fig. 1(d). While the cells flow into the cell-patterning area above the concentric-stellate-tip array electrodes, the cells will be manipulated by the balance force contributed from the field-induced positive DEP and the hydrodynamic force. By applied the adequate ac potential with the suitable input flow rate, the spatial gradients of electric fields are generated to manipulate the cells. The spatially randomly distributed cells are, then, guided to the stellate-tips, string into pearl-chain patterns by positive DEP effect, are snared from individual local strings to a net, and finally form the radial pearl-chain patterns, which approximate the radial cell pattern as seen in the classic lobule [Fig. 1(a) and (e)].Numerical simulation of field-enchanced dielectrophoresis
To verify the DEP effect induced by the electric-field gradients of concentric-stellate-tip array electrodes for predicting the cell-patterning performance, a commercial finite element software CFD-ACE+ (CFDRC, Huntsville, AL) is used to simulate the steady-state electric field, which dominates the field-induced DEP force used in this work.Fig. 2(a) and (b) show the simulation results for the root mean square of ac electric field (Esquare) for the concentric-ring-array design (without tips) and the concentric-stellate-tip-array design (with stellate-tips), respectively. The operating ac potential is set up as 5 V peak–peak at 1 MHz (more details will be addressed later). Due to the symmetrical property in the two electrode designs, the simulation is done only for 1/8 of a full round pattern-unit to simplify numerical computation. For the concentric-ring-array design without the tips, the simulation result [Fig. 2(a)] shows that the Esquare distribution is non-uniform and axially symmetric under the ac potentials applied to the ring electrodes. The electric field near the inner rings is higher than the outer rings. This pure concentric-ring-array could provide a stable region for DEP manipulation. Theoretically, cells can be guided by positive DEP phenomenon and, then, align into the pearl-chain arrays between the adjacent electrode rings. However, the pear-chain cells will be randomly distributed between the adjacent electrode rings because of lacking of tangential DEP guiding force as the comparison shown in Fig. 2(a) and (b). For the concentric-ring-array electrodes, the nearby cells, initially, are mostly attracted to the interior ring electrodes with random distribution, because the smaller curvature radius results in the stronger electric field but the smooth ring electrodes lack of tangential DEP-force control. Then, some of the following cells are attracted by DEP force and attach to the initially snared cells. However, some more following cells would still be driven towards the electrode rings and accumulate in the vacant regions which have not been occupied by cells. Finally, the whole cell-patterning area will be fully occupied by cells without any spatial pattern.
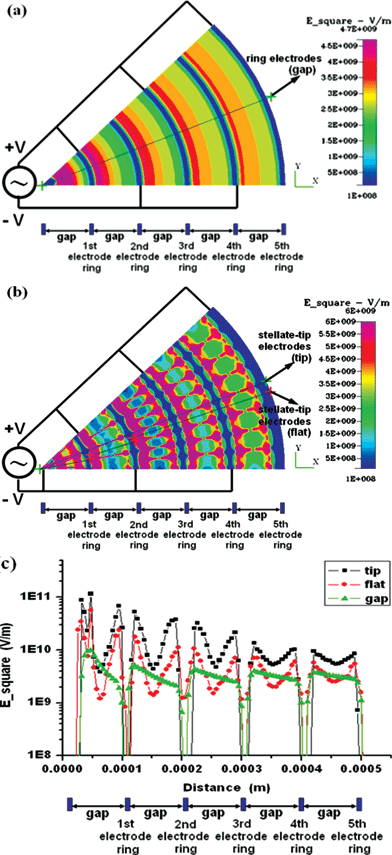 | ||
| Fig. 2 Numerical simulation of DEP induced by applying the potentials of 5 V peak–peak at 1 MHz for the concentric-ring-array electrodes and for the concentric-stellate-tip array electrodes. (a) The distribution of the root mean square of ac electric field (Esquare) for the concentric-ring-array electrodes. (b) The distribution of Esquare for the concentric-stellate-tip-array electrodes. (c) The numerical comparison for the Esquare versus the radial distance from the center of ring circles. The triangle marked line represents the Esquare values along the radial line as shown in (a) for concentric-ring-array electrodes. The square and the diamond marked lines represent the Esquare values along the radial line crossing the stellate-tips and the smooth electrode ring, respectively, as shown in (b). | ||
For the concentric-stellate-tip-array design, Fig. 2(b) simulates the distribution of Esquare. The stellate-tips design repeatedly provides numerous local gradient maxima of electric field not only along the radial direction but also tangentially between adjacent stellate-tip electrode rings. The area between two counter tips at each adjacent electrode pair (tip-to-tip) has a locally maximum electric field. Due to the positive DEP effect, the cells, under appropriate ac potentials, could be guided from the lower electric-field region to the higher electric-field region. As a result, cells could be attracted by the field-induced DEP and form the precise radial pearl-chain pattern from the tip to the counter tip along the orientation of the electric line. In order to analyze the difference and distribution between the two electrode designs to obtain more clear physical insight, the intensity profiles of the Esquare on the electrode plane (z = 0) along the radial line depicted in Fig. 2(a) and (b), are simulated and shown in Fig. 2(c). The electric field generated by the pure concentric-ring-array electrodes is non-uniform and axially symmetric, and decreases gradually from inner ring to outer ring with piecewise gentle slopes, as the triangle-marked line shown in Fig. 2(c). The gentle slopes indicate that the gradient variation of the Esquare induced by concentric-ring-array electrodes results in the weaker DEP forces. However, through the concentric-stellate-tip array design, the stellate-tips not only produce the local maximum electric fields near the stellate tips but also highly enhance the gradients of the electric fields, which is verified by the steep slopes of the square-marked piecewise line as shown in Fig. 2(c). Besides, as shown in Fig. 2(b) and the diamond-marked line in Fig. 2(c), the smooth sections on the concentric-stellate-tip array electrodes have the lower electric fields and offer the local minimum electric field, which tangentially pushes the cells towards the line between the counter tips, when the positive DEP potentials are applied. Hence, the positive DEP in our concentric-stellate-tip array design may more precisely provide the net attractive force to snare cells in pearl-chain pattern along the radial electric-field lines between the nearest counter stellate-tips as shown in Fig. 2(b).
MEMS fabrication and material preparation for cell patterning
Microfabrication process
The cell-patterning chip, which takes advantage of enhanced DEP force precisely in space, was micromachined using IC photolithography process, which is summarized and illustrated in Fig. 3(a)–(f). A silicon substrate was prepared by using the piranha clean technique. The chamber and microchannel height of 100 µm was defined by using SU-8 negative photo resist [Fig. 3(a)]. After the elastomer base and the curing agent of PDMS (Sylgard 184, Dow Corning, USA) were mixed together (10 ∶ 1), the mixture was poured onto the silicon-PR mold prior to a degassing step and cured at 90 °C for one hour. Then, holes of 1 mm were mechanically punched through the cured detached PDMS top cover for the purpose of fluidic connections to outside tubing [Fig. 3(c)]. The concentric-stellate-tip electrodes were micromachined on a glass substrate by the photolithography process with the E-Gun evaporation of a 200Å/2000Å titanium/platinum layer. Then, the fine electrode patterns were obtained via the lift-off process [Fig. 3(d)]. After the oxygen plasma treatment on both the glass substrate and the PDMS top cover [Fig. 3(e)], the two parts were aligned and bonded together [Fig. 3(f)]. Finally, plastic tubes were connected to the sample inlets and outlets from the punched holes. The final whole cell-patterning biochip and the zoom-in pictures were shown in Fig. 3 (g)–(i). | ||
| Fig. 3 The microfabrication process of our cell-patterning chip and the final chip pictures. (a) SU-8 negative photoresist is used to define microchannels and pattering chamber (100 µm high). (b) PDMS is micromolded to serve as the transparent top cover. (c) The fluidic connections are mechanically punched through on the PDMS top cover. (d) The concentric-stellate-tip array electrodes are micromachined on the glass substrate. (e) The poly-D-lysine is coated on the glass substrate and the oxygen plasma treatment is applied on the PDMS mold for surface modification. (f) The glass is bonded to the PDMS top cover. (g) The picture of the final cell-patterning chip. (h) The SEM image of the detail electrode geometry. (i) The close-view of the concentric ring electrodes with stellate-tips. | ||
Cell culture and media
Human liver cell line, HepG2 (ATCC, HB8065), was maintained at 37 °C with 95%/5% air/CO2 in Iscove's modified Dulbecco's medium (IMDM, Gibco-BRL, NY) containing 10% (v/v) heat-inactivated fetal bovine serum (FBS, Biological Industries, Israel) and antibiotics (100 U ml−1 penicillin and 100 U ml−1 streptomycin, Sigma-Aldrich Co., MO). Human umbilical vein endothelial cells (HUVECs) were prepared as previously described39 and maintained in M200 medium supplemented with low serum growth supplement (LSGS, both from Cascade Biologics Inc.). These two types of cells are utilized to demonstrate the rapid on-chip patterning of heterogeneous cells in this paper.Cell preparation for DEP manipulation
Prior to the on-chip cell patterning via DEP for the first type of cells, HepG2 cells were harvested from sub-confluent cultures by trypsin/EDTA (Sigma) and re-suspended in the DEP-manipulating buffer (8.5% sucrose and 0.3% dextrose in ddH2O; conductivity: 10 mS m−1) to result in a final concentration of 5 × 106 cells ml−1. The conductivity of medium was measured via a digital conductimeter (330i, WTW, Germany). For heterogeneous-cells patterning, HepG2 cells and HUVECs were pre-labeled with biocompatible fluorescent dyes, DiO (green) and DiI (red), for the identification at the excitation/emission wavelengths of 488/520 nm and 530/565 nm, respectively.40Surface modification of the cell-patterning chamber for cell-adhesion enhancement
While the low conductivity of DEP-manipulating buffer is unsuitable for cell adhesion, the surface pre-treatment of the cell-patterning biochip is required to achieve sufficient cell adhesion and growth. For this purpose, the auxiliary bonding area outside the cell-patterning area of the chip was, first, sealed with the tape. Then, the cell-patterning area of the chip was dipped in a 5% (w/v) poly-D-lysine (Sigma) solution at 37 °C for 12 hours to adsorb a thin layer of positively charged molecules. Then, the chip was washed by flowing ddH2O for 10 minutes to ensure that no unbound poly-D-lysine releases on the chip and influences the cell-patterning condition by extra conductivity during the positive DEP manipulation.Experimental setup for cell-patterning experiments
A syringe pump (SP230IW, WPI, Florida, USA) was used to pump and regulate the flow streams of the fluid. A function generator (33120A, Agilent) was used to generate the out-of-phase ac voltage sources required for the DEP operation on the concentric-stellate-tip array electrodes in our chip. The input electrical signals were monitored by using an oscilloscope (54624A, Agilent, USA). The motions and displacements of cells were monitored via an inverted microscope (BX51, Olympus, Tokyo, Japan) and the images and movies were captured and recorded via a digital CCD-camera connected to a laptop computer.Cell adhesion, growth and viability assessment in the cell-patterning chamber
A programmable adjustment of liquid environment for the cell-patterning chamber was performed carefully as described below to satisfy the different requirements of medium compositions at each step.Results and discussion
The parameter setup for DEP operation
An ac electric field imposed on a freely suspended cell would induce a dipole moment within the cell and result in a potential difference across the plasma membrane. This induced voltage might lead to the membrane permeabilization due to an electrical breakdown on the plasma membrane when the membrane potential difference approximately exceeds 1 V at room temperature.42 Exposing biological cells to such a high electric-fields environment might lead to a variety of profound biochemical and biophysical effects, such as apoptosis and cell-lysis.43 Besides, another challenge in this cell-patterning research via DEP manipulation is that the normal cell-culture buffer usually has a high electrical conductivity and is unsuitable for DEP manipulation. The normal biological buffer with a high electrical conductivity was accompanied by two drawback effects under normal DEP operation: (i) the heating of the solution; (ii) the electrolytic process (gas bubble formation). Therefore, the low-conductivity medium, which could also decrease the induced transmembrane potential, is favorable for the DEP manipulation of the cells because field-induced apoptosis hardly occurs under low conductivity medium. In addition, since a high-frequency ac potential (above sub-megahertz) could alleviate the induced potential more than a low-frequency one,43 the manipulation of the cells was operated in a low conductivity medium with an ac potential of 1 MHz to provide a soft electric environment and minimize the stress on the delicate cells. Moreover, it has been reported that the mammalian cells can sustain prolonged exposure (40 minutes or longer) to the electric field of less than 104 V m−1 without the loss of viability.44,45 Under the electric field of about 5 × 104 V m−1, temporary membrane electro-permeabilization occurs but the cells will reseal and maintain viability after such a loading if the suspension conditions are gentle. However, above the electric field of 2 × 105 V m−1, irreversible disruption of cell membranes occurs. After long-term DEP and cell-viability experiments, we compromise between a strong DEP force and a good cell viability during/after the DEP manipulation to set up the DEP potential of 5 V peak–peak at 1 MHz with the gentle low-conductivity medium described above to relax the environmental stress for cells. More details will be addressed in the next section.On-chip in-parallel cell-patterning demonstration
Human liver cells, HepG2, with the size of about 10–15 µm in diameter, were used for our cell-patterning experiments demonstrated in this paper. Before the cells were introduced into the bio-chip, the fluidic network was filled with the buffer of 8.5% sucrose plus 0.3% dextrose, which provides the isotonic supporting medium with a low conductivity of about 10 mS m−1. The cells, HepG2, were then introduced with the medium (the cell concentration: 5 × 106 cells ml−1) from the inlet via a syringe pump (SP 230IW, WPI, USA) with a flow rate of 5–30 µl min−1. After the flow got steady and the cells were distributed over the cell-patterning chamber, the DEP voltage was turned on for parallel cell manipulation. The ac voltages (1–10 V) with the frequency varying from 100 Hz to 10 MHz were applied to the electrode array in our chip for characterizing the positive and the negative DEP phenomena of the liver cells. For the sake of avoiding electrolysis and cell lyses, the potential of 5 V peak–peak should be above the frequency of 10 KHz based our experimental observation. With the low-conductivity medium of 10 mS m−1 used in our demonstration, experiments showed that the negative DEP phenomenon appeared on the liver cells (HepG2) at low-frequency while the positive DEP phenomenon appeared at high frequency. The crossover frequency of the DEP phenomena for the liver cells took place at about 12 KHz. In this research, we took advantage of positive DEP force, which attracts to the high electric-field area, to generate the desired radial pearl-chain cell pattern which mimics the lobular morphology of real liver tissue. As addressed in previous section, a compromise of 5 V peak–peak and 1 MHz potential was utilized to approach both strong DEP force and good cell viability in our cell-patterning demonstration.A time sequence of micrograph images shown in Fig. 4 demonstrate the cell patterning due to the non-uniform electric field in our chip with the applied ac potentials of 5 V peak–peak at 1 MHz. Fig. 4(a) shows the cell-patterning chamber, which was filled with pure DEP buffer before cells were introduced. Fig. 4(b) shows the random distribution of liver cells, which were pumped in with the buffer medium of 20 µl min−1 flow rate without DEP cell-patterning manipulation, over the glass substrate of the cell-patterning chamber. After the DEP field was turned on, the stellate-tip electrodes provided local maximum electric-field gradients between the counter electrode tips at the adjacent electrode annuli to act as the primers for the cell aggregation of cell patterning. The HepG2 cells were first trapped in parallel at the stellate tips and, then, aggregated in line starting from the stellate tips as shown in Fig. 4(c) (five seconds after turning on DEP voltages). With the continuous supply of “fresh cells” from the surrounding medium, the cell-chain length increased rapidly with time by the enhanced field-induced DEP. Cell chains, then, lined up and between counter electrodes at the adjacent electrode rings. Fig. 4(d) shows the radial pearl-chain-like pattern of liver cells which were snared from individual local strings along the enhanced electric-field lines to the net mimicking the pattern of the classic hepatic lobule.
 | ||
| Fig. 4 A time sequence of images for positioning liver-cells (HepG2) in a non-uniform DEP force field under the DEP voltage of 5 V peak–peak at 1 MHz. (a) The cell-patterning chamber was filled with pure buffer before HepG2 cells were introduced. (b) The liver cells were randomly distributed before the DEP manipulation voltages were turned on. (c) The image is recorded on the fifth second after the DEP manipulation voltages were turned on. The cells were snared at the stellate tips and aggregated in line along the enhanced electric-field lines in parallel. (d) The image was recorded at the 45th second after the DEP manipulation voltages were turned on. The cell chains lined up in parallel to form the biomimetic radial pattern. On (b) and (c), the background medium flows downward. | ||
In situ cell-viability assessment for DEP cell patterning
In order to observe the viability of the patterned HepG2 cells for the further applications such as the fundamental research of liver metabolic function in vitro, the liver toxicity for drug applications and the tissue-reconstruction for tissue engineering,9–12 an in situ fluorescence-staining method via FDA/EtBr assay is utilized in this paper. The live and the dead cells can be monitored and distinguished simultaneously via this FDA/EtBr cell-viability assay method. The results are shown in Fig. 5. Fig. 5(a) shows that the HepG2 cells were snared and the pearl-chain pattern of the HepG2 cells formed along the enhanced electric-field lines under the applied DEP voltages of 5 V peak–peak at 1 MHz. The image in Fig. 5(a) was recorded after the pure DEP buffer of 20 µl min−1 flow rate (without cells) became steady and flushed away the extra cells out of the cell-patterning chamber under the continuous DEP operation. The experiment results show that the DEP force can trap and hold liver cells against the flow rate up to about 200 µl min−1. After the above DEP operation on liver cells continued for 5 minutes, the FDA/EtBr dyes were injected into cell-patterning chamber from the inlet. The cell-viability test result via the FDA/EtBr fluorescence assay is shown in Fig. 5(b). The fluorescence microscope image of on-chip patterned liver cells simultaneously shows both the viable cells (stained with green fluorescence) and the dead cells (stained with red fluorescence). The high-percentage cell survival rate of above 95% is observed on our cell-patterning chip for one-hour continuous DEP manipulation with the conditions of the iso-osmotic low conductive buffer and the DEP voltages of 5 V peak–peak at 1 MHz. | ||
| Fig. 5 In situ cell-viability demonstration for the on-chip cell patterning of human hepatoma cells. (a) HepG2 cells aggregated together and aligned into the pearl-chain pattern along the enhanced electric-field lines under the DEP operation of 5 V peak–peak at 1 MHz. (b) The fluorescent microscope image for in situ FDA/EtBr cell-viability assay. The results show a cell survival rate as high as 99%. | ||
Enhancement of cell adhesion via poly-D-lysine coating and medium replacement
For the positive DEP manipulation, the low-conductivity medium is required to ensure the sufficient DEP-force for snaring/positioning/patterning cells. The inherent incompatibility between the DEP-manipulating buffer (low conductivity) and mammalian cell culture medium (high conductivity with lots of ions) is one of the main challenges in this research. The isotonic sucrose/dextrose buffer is excellent for DEP-manipulation on our cell-patterning chip but it is unsuitable for the cell adhesion as well as the cell growth. Moreover, the cell-viability rate decreases rapidly when the HepG2 cells are maintained in such a DEP-manipulating buffer for more than one or two hours. In order to enhance both the cell survival rate and the cell growth rate, the snared/patterned cells must adhere to the chamber substrate as soon as the cells are snared and trapped onto the desired position via the enhanced DEP manipulation. Then, the buffer needs to be replaced by the fresh normal culture medium (IMDM containing lots of ions as described earlier) to minimize the environmental stress and to support the cell long-term growth.In order to enhance the cell-adhesion, we coated the substrate of the cell-patterning chamber with poly-D-lysine via soaking the chamber in the poly-D-lysine solution of 100 µg ml−1 for 12 hours. Then the process was followed by washing the chip three times via pumping in the DEP-manipulating buffer before the chip was used for cell patterning. The positive-charged poly-D-lysine was used to enhance the cell-attachment between the cells and the glass chip substrate. We started the demonstration of cell-adhesion enhancement from gradually replacing the original DEP-manipulating buffer with the cell-free DEP-manipulating buffer. The cell-free DEP-manipulating buffer was supplemented with 5 mM Ca2+ and 5 mM Mg2+ to enhance the cell-attachment capability after cell patterning was finished via the DEP manipulation. After part of the original DEP-manipulating buffer had been replaced by the above cell-free buffer with a flow rate of 10 µl min−1 for 15 minutes, the adhesive force between patterned cells and poly-D-lysine coated surface was strong enough to keep patterned cells in place. Based on our experimental observation, the holding adhesion-force between patterned cells and substrate in our device is strong enough to resist the flushing force due to the flow pumping up to a flow rate of 500 µl min−1 without washing any cell away. During the adhesion-enhancement/medium-replacing process, the additional Ca2+ and Mg2+ ions from the fresh cell-free DEP-manipulating buffer reduced the DEP force due to the increase of overall conductivity in the cell-patterning chamber. However, the additional Ca2+ and Mg2+ ions could enhance the adhesion force not only between the cells and the substrate of cell-patterning chamber but also among the cells via the activating molecules of cell adhesion, which includes cadherins and integrins.40 The setup of experimental parameters such as the medium selection, the flow rate, the timing and the DEP potential is compromised among the cell viability, the cell adhesion and the DEP force for successfully approaching the on-chip heterogeneous liver-cells patterning which mimics the radial pattern of the real hepatic lobule.
In addition to the demonstration of on-chip heterogeneous liver-cells patterning, we went through the following experimental observation to verify the long-term cell viability and growth after the cells experienced a period of DEP force manipulation. After the 15-minute adhesion-enhancement process, the DEP voltages were turned off and the normal cell-culture medium (IMDM as described earlier) was pumped in for further cell viability and growth studies. After one-hour on-chip cell culture under the normal culture medium without DEP manipulation, the patterned HepG2 liver cells still arrange in radial pearl-chain pattern along the orientation of tip-to-tip. The pearl-chain-cell lines now look a little bit twisted with the larger cell size from the top view. This result demonstrates that the liver cells are live, grow and start to spread and crawl on the substrate of the cell-patterning chamber. The cell adhesion/growth behavior not only shows that the cells are viable but also provide the access of further proliferation, differential and cell–cell interaction for approaching further functional liver tissue.
Heterogeneous integration of HepG2 cells and HUVECs for liver-cells patterning
To mimic the classical lobule organization of liver tissue for the development of functional tissue, heterogeneous integration of the human liver cells (HepG2) and the human umbilical vein endothelial cells (HUVECs) were patterned and demonstrated via the enhanced specific DEP design and manipulation on our biochip. There are still two more challenges in this research: (i) the accurate dynamic simulation for individual-cells dynamics under the time-varying DEP force field caused by multi-cells dynamics; (ii) the appropriate snaring of both HepG2 cells and HUVECs to form the desired radial HepG2 cells pattern and the alternate radial HUVECs pattern mimicking the sinusoid-like vascular endothelial lining cells appearing in the classic real hepatic lobule. The moving/static cells above the electrodes will introduce the dynamical/steady redistribution/reshaping of DEP electric field. This is part of reason that the patterned cells (shown in Fig. 4, 5 and 6) did not precisely follow the predicted result of cell-free electric-field simulation. Anyway, the much more precise cell pattern is not necessary to be pursued based on the observation of real functional hepatic lobule. In our case, the second type of cells, HUVECs, need to be positioned after the first type of cells, HepG2, are snared in radial pattern. This heterogeneous integration makes this research challenging from the engineering aspect. The more challenge on the accurate dynamic simulation is that the cells are deformable, electrically non-uniform and complicated multi-shells. Thus, to simplify the numerical problems, we only presented the numerical simulation for the electric fields of our DEP manipulation under the conditions of steady-state and cell-free medium. From the simplified simulation results, we obtain physical insight to improve our cell-patterning chip design and verify our design via the experimental demonstration. | ||
| Fig. 6 On-chip heterogeneous-cells patterning demonstration. (a) The first group of HepG2 cells are snared and patterned in radial pearl-chains via the concentric-stellate-tip array electrodes with the in-parallel DEP manipulation of 5 V peak–peak at 1 MHz. The image is recorded under white light. (b) The on-chip demonstration for the rapid heterogeneous-integration patterning of HepG2 cells (green fluorescence) and HUVECs (red fluorescence). Both HepG2 pearl-chains and HUVECs pearl-chains are snared and align in the radial patterns via the enhanced field-induced DEP manipulation. The alternate radial patterned HUVECs mimic the shape and the function of sinusoid-like vascular endothelial lining cells that are shown in the classic real hepatic lobule. (c) The fluorescent control group with the two types of cells randomly distributed over the cell-patterning chamber without DEP manipulation. In (a), (b), and (c), the medium flows from the right to the left. | ||
Fig. 6 shows the results of on-chip heterogeneous liver-cells patterning demonstration. The first group of DiO-labeled HepG2 cells was patterned with the same procedure as described earlier [Fig. 6(a)]. After the Ca2+/Mg2+ supplemented non-DEP-manipulating buffer was injected with the flow rate of 10 µl min−1 to replace the original DEP-manipulating buffer, the cell-patterning chip was held steadily for 15 minutes to allow good cell adhesion for the patterned HepG2 cells. Then, unattached cells were washed away by the DEP buffer of 200 µl min−1 flow rate. The DiI-labeled HUVECs were then pumped in with the DEP operation on. At this moment, the snared/patterned HepG2 cells had occupied the area of local electric-field maximum to form the lobule-mimetic radial pattern and reshaped the electric field. The incoming snared HUVECs could only be attracted to the available local electric-field maxima via continuous DEP manipulation. Fig. 6(b) shows the result that the HUVECs were snared and filled into the left vacancy to form the additional alternate radial pearl-chain array, which mimics the shape and the function of sinusoid-like vascular endothelial lining cells in the classic real hepatic lobule. The fluorescence image [Fig. 6(b)] shows the result of the rapid on-chip heterogeneous integration of live HepG2 cells (green) and live HUVECs (red) via our enhanced field-induced DEP trap design. The same procedure of culture-medium replacement was performed but the HepG2-HUVEC co-culture medium was changed to the M200 (described earlier). The further studies on cell–cell interaction, growth, drug tests, and 3-D cell patterning are undergoing in our group. For our present chip design, several thousands of hepatic cells could be snared and patterned to mimic the lobular pattern in vitro over the 2-D patterning region of about 0.8 mm2. Fig. 6(c) shows the experimental control group with the two types of cells randomly distributed over the cell-patterning chamber without DEP manipulation. Compared to the experimental control group, the heterogeneous integration of HepG2 cells and HUVECs via our chip design exhibits significant radial pattern with the fine resolution to provide the intimate contact for cell-to-cell interaction and development. This technique, after further development, could be applied to the fields of tissue engineering and drug development.
Conclusions
A rapid, heterogeneous-cell patterning microfluidic chip facilitates the construction of liver tissue in vitro, via the enhanced DEP trap design, with in-parallel rapid manipulation, stable control, high cell viability, and cell modulation. The design of concentric-ring array electrodes combined with stellate tips could enhance the inhomogeneous field effect to snare individual cells much more precisely to form the desired biomimetic pattern. The original randomly distributed liver cells in our chip could be manipulated in parallel and aligned into the desired radial pearl-chain array to form the biomimetic radial pattern, which mimics the cell-pattern morphology of real liver tissue, with good cell viability after cell-patterning DEP manipulation. Heterogeneous integration of liver-cell patterning is demonstrated on our present bio-chip, with several thousands of HepG2 cells and HUVECs snared and patterned on the patterning area of about 0.8 mm2. Some further studies on cell–cell interaction, growth, drug tests, and 3-D cell patterning are in progress in our group. High-resolution cell pattering methods capable of controlling the heterogeneous cells as well as the cell–cell interactions, which potentially modulate the cell behaviour, would enable reproducible control over the cellular microenvironment and could benefit the maintenance of cell functions in in vitro physiological systems. This proposed cell-patterning chip could be applied to biological research, biomedical investigation and tissue engineering.Acknowledgements
This research was financially supported by the Nano-technology Research Program of the University System of Taiwan, the National Science Council under grant NSC-94-2215-E-007-001, and the Veteran General Hospitals University System of Taiwan Joint Research Program under grant VGHUST94-G6-06-3. The authors thank the Center for Nano-Science and Technology at the University System of Taiwan, the Semiconductor Research Center and the National Nano Device Laboratory for the micro-fabrication facility support of these common labs. We also thank Prof. Long Hsu, Prof. Hwei-Ling Peng and Prof. Tri-Rung Yew for helpful comments.References
- U. A. Stock and J. P. Vacanti, Tissue engineering: current state and prospects, Annu. Rev. Med., 2001, 52, 443–451 CrossRef CAS.
- L. G. Griffith and G. Naughton, Tissue engineering: current challenges and expanding opportunities, Science, 2002, 295, 1009–1014 CrossRef CAS.
- B. Palsson, J. A. Hubbell, R. Plonsey and J. D. Bronzino, Principles and Applications in Engineering Series, Tissue Engineering, CRC Press, Boca Raton, FL, 2003 Search PubMed.
- A. Atala and R. Lanza, Methods of Tissue Engineering, Academic Press, San Diego, CA, 2001 Search PubMed.
- J. W. Allien and S. N. Bhatia, Engineering Liver Therapies for the Future, Tissue Eng., 2002, 8(5), 725–737 CrossRef.
- H. Andersson and A. V. D. Berg, Microfabrication and microfluidics for tissue engineering: start of the art and future opportunities, Lab Chip, 2004, 4, 98–103 RSC.
- A. Curtis and M. Riehle, Tissue engineering: the biophysical background, Phys. Med. Biol., 2001, 46, 47–65.
- J. J. Pancrazio, J. P. Whelan, D. A. Borknolder, W. Ma and A. Stenger, Development and Application of Cell-Based Biosensors, Ann. Biomed. Eng., 1999, 27, 697–711 CrossRef CAS.
- K. Bhadriraju and C. S. Chen, Engineering cellular microenvironments to improve cell-based drug testing, Drug Discovery Today, 2002, 7(11), 612–620 CrossRef CAS.
- A. S. Rudolph, Cell and tissue based technologies for environmental detection and medical diagnostics, Biosens. Bioelectron., 2001, 16, 429–431 CrossRef CAS.
- W. M. Saltzman and W. L. Olbricht, Building Drug Delivery into Tissue Engineering, Nat. Rev. Drug Discovery, 2002, 1, 177–186 CrossRef CAS.
- D. W. Huntmacher, Scaffold design and fabrication technologies for engineering tissues-state of the art and future perspectives, J. Biomater. Sci., Polym. Ed., 2001, 12(1), 107–124 CrossRef CAS.
- V. L. Tsang and S. N. Bhatia, Three-dimensional tissue fabrication, Adv. Drug Delivery Rev., 2004, 56, 1635–1647 CrossRef.
- R. S. McCuskey, Morphological mechanisms for regulating blood flow through hepatic sinusoids, Liver Int., 2000, 20, 3–7 Search PubMed.
- S. Bhatia, M. Yarmusch and M. Toner, Controlling cell interactions by micropatterning in co-cultures:hepotocytes and 3T3 fibroblasts, J. Biomed. Mater. Res., 1997, 34, 189–199 CrossRef CAS.
- K. Headly, C. Thomas, A. Rezania, J. Kim, P. McKeown, B. Lom and P. Hockberger, Kinetics of bone cell organization and mineralization on materials with patterned surface chemistry, Biometerials, 1996, 17, 195–208 Search PubMed.
- M. Mrksich, L. E. Dike, J. Tein, D. E. Ingber and G. M. Whitesides, Microcontact Printing on Pattern the Attachment on Mammalian Cells to Self-Assembled Monolayers of Alkanethiolates on Transparent Films of Gold and Silver, Exp. Cell Res., 1997, 235, 305–313 CrossRef CAS.
- S. Zhang, L. Yan, M. Altman, M. Lässle, H. Nugent, F. Frankel, D. A. Lauffenburger, G. M. Whitesides and A. Rich, Biological surface engineering: a simple system for cell pattern formation, Biomaterials, 1999, 20, 1213–1220 CrossRef CAS.
- D. T. Chiu, N. L. Jeon, S. Huang, R. S. Kane, C. J. Wargo, I. S. Choi, D. E. Ingber and G.. M. Whitesides, Patterned deposition of cells and protein onto surfaces by three-dimensional microfluidic systems, Proc. Natl. Acad. Sci. U. S. A., 2000, 97(6), 2408–2413 CrossRef CAS.
- S. Takayama, E. Ostuni, P. LeDuc, K. Naruse, D. E. Ingber and G. M. Whitesides, Laminar flow: Subcellular positioning of small molecules, Nature, 2001, 411, 1016 CrossRef CAS.
- Y. Nahmias, R. E. Schwartz, C. M. Verfaillie and D. J. Odde, Laser-guided direct writing for three-dimensional tissue engineering, Biotechnol. Bioeng., 2005, 92(2), 129–136 CrossRef CAS.
- H. A. Pohl, Dielectrophoresis, Cambridge University Press, Cambridge, UK, 1978 Search PubMed.
- M. P. Hughes, Nanoelectromechanics in Enginnering and Biology, CRC Press, Boca Raton, FL, 2003 Search PubMed.
- N. G. Green, H. Morgan and J. J. Milner, Manipulation and trapping of sub-micro bioparticles using dielectrophoresis, J. Biochem. Biophys. Methods, 1997, 35, 89–102 CrossRef CAS.
- T. Muller, G. Gradl, S. Howitz, S. Shirley, T. Schnelle and G. Fuhr, A 3-D microelectrode system for handling and caging single cells and particles, Biosens. Bioelectron., 1999, 14, 247–256 CrossRef CAS.
- P. R. C. Gascoyne, X. B. Wang, Y. Huang and F. F. Becker, Dielectrophoresis separation of cancer cells from blood, Trans. Ind. Appl., 1997, 33(3), 670–678 Search PubMed.
- H. Morgan, M. P. Hughes and N. G. Green, Separation of subparticles by dielectrophoresis, Biophys. J., 1999, 77, 516–525 CrossRef CAS.
- S. Choi and J. K. Park, Microfluidic system for dielectrophoretic separation based on a trapezoidal electrode array, Lab Chip, 2005, 5, 1161–1167 RSC.
- X. B. Wang, J. Yang, Y. Huang, J. Vykoukal, F. F. Becker and P. R. C. Gascoyne, Cell separarion by dielectrophoretic field-flow fractionation, Anal. Chem., 2000, 72, 832–839 CrossRef.
- S. Fiedler, S. G. Shirley, T. Schnelle and G. Fuhr, Dielectrophoretic sorting of particles and cells in a microsystem, Anal. Chem., 1998, 70, 1909–1915 CrossRef CAS.
- J. H. Nieuwenhuis, A. Jachimowicz, P. Svasek and M. J. Vellekoop, Optimization of microfluidic particles sorters based on dielectrophoresis, Sens. J., 2005, 5(5), 810–816 Search PubMed.
- P. R. C. Gascoyne and J. V. Vykoukal, Dielectrophoresis-based sample handling in general-purpose programmable diagnostic instruments, Proc. IEEE, 2004, 92(1), 22–40 CrossRef CAS.
- T. Matsue, N. Matsumoto and I. Uchida, Rapid micropatterning of liver cells by repulsive dielectrophoretic force, Electrochim. Acta, 1997, 42, 3251–3256 CrossRef CAS.
- M. Frenea, S. P. Faure, B. L. Pioufle, P. Coquet and H. Fujita, Positioning living cells on a high-density electrode array by negative dielectrophoresis, Mater. Sci. Eng., C, 2003, 23, 597–603 CrossRef.
- Z. Yu, G. Xiang, L. Pan, L. Huang, Z. Yu, W. Xing and J. Cheng, Negative dielectrophoretic force assisted construction of ordered neuronal networks on cell positioning bioelectronic chips, Biomed. Microdevices, 2004, 6(4), 311–324 CrossRef CAS.
- D. S. Gray, J. L. Tan, J. Voldman and C. S. Chen, Dileectrophoretic registration of living cells to a microelectrode array, Biosens. Bioelectron., 2004, 19, 771–780 CrossRef CAS.
- D. R. Albrecht, V. L. Tsang, R. L. Sah and S. N. Bhatia, Photo- and electropatterning of hydrogel-encapsulated living cell array, Lab Chip, 2005, 5, 111–118 RSC.
- B. Alp, J. S. Andrews, V. P. Mason, I. P. Thompson, R. Wolowacz and G. H. Markx, Building structured biomaterials using AC electrokinetics, IEEE Eng. Med. Biol. Mag., 2003, 22(6), 91–97 CrossRef.
- E. A. Jaffe, R. L. Nachman, C. G. Becker and C. R. Minick, Culture of human endothelial cells derived from umbilical veins, identification by morphologic and immunological criteria, J. Clin. Invest., 1973, 52, 2745–2756 CrossRef CAS.
- R. Z. Lin, L. F. Chou, C. C. M. Chien and H. Y. Chang, Dynamic analysis of hepatoma spheroid formation: roles of E-cadherin and β1-integrin, Cell Tissue Res., 2006, 324(3), 411–422 CrossRef CAS.
- C. T. Ho, R. Z. Lin, H. Y. Chang and C. H. Liu, Micromachined T-switches for cell sorting applications, Lab Chip, 2005, 5, 1248–1258 RSC.
- U. Zimmermann, Electromanipulation of Cells, CRC Press, London, 1996, pp. 1–106 Search PubMed.
- U. Zimmermann, U. Friedrich, H. Mussauer, P. Gessner, K. Hämel and V. Sukhorukov, Electromanipulation of mammalian cells: fundamentals and application, IEEE Trans. Plasma Sci., 2000, 28(1), 72–82 CrossRef CAS.
- X. J. Wang, J. Yang and P. R. C. Gascoyne, Role of peroxide in ac electric field exposure effects on friend murine erythroleukemia cells during dielectrophoretic manipulations, Biochim. Biophys. Acta, 1999, 1426(1), 53–68 CrossRef CAS.
- G. Fuhr, R. Hagedorn, R. Glaser, J. Gimsa and T. Müller, J. Electr., 1987, 6, 49–69 Search PubMed.
Footnote |
| † C.-T. Ho and R.-Z. Lin contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2006 |
