An actuated pump on-chip powered by cultured cardiomyocytes†
Yo
Tanaka
ab,
Keisuke
Morishima
bcd,
Tatsuya
Shimizu
be,
Akihiko
Kikuchi
be,
Masayuki
Yamato
be,
Teruo
Okano
be and
Takehiko
Kitamori
*abc
aDepartment of Applied Chemistry, School of Engineering, The University of okyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: kitamori@icl.t.u-tokyo.ac.jp; Fax: +81-3-5841-6039; Tel: +81-3-5841-7231
bCore Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency, Kawaguchi, Saitama 332-0012, Japan
cMicro Chemistry Group, Optical Science Laboratory, Kanagawa Academy of Science and Technology (KAST), 3-2-1 Sakado, Takatsu-ku, Kawasaki, Kanagawa 213-0012, Japan
dDepartment of Mechanical Systems Engineering, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588, Japan
eInstitute of Advanced Biomedical Engineering and Science, Tokyo Women's Medical University, 8-1 Kawada-cho, Shinjuku-ku, Tokyo 162-8666, Japan
First published on 25th January 2006
Abstract
Cellular functions are frequently exploited as processing components for integrated chemical systems such as biochemical reactors and bioassay systems. Here, we have created a new cell-based microsystem exploiting the intrinsic pulsatile mechanical functions of cardiomyocytes to build a cellular micropump on-chip using cardiomyocyte sheets as prototype bio-microactuators. We first demonstrate cell-based control of fluid motion in a model microchannel without check valves and evaluate the potential performance of the bio-actuation. For this purpose, a poly(dimethylsiloxane) (PDMS) microchip with a microchannel equipped with a diaphragm and a push-bar structure capable of harnessing collective cell fluid mechanical forces was coupled to a cultured pulsating cardiomyocyte sheet, activating cell-based fluid movement in the microchannel by actuating the diaphragm. Cell oscillation frequency and correlated fluid displacement in this system depended on temperature. When culture temperature was increased, collective cell contraction frequency remained cooperative and synchronous but increased, while displacement was slightly reduced. We then demonstrated directional fluid pumping within microchannels using cantilever-type micro-check valves made of polyimide. A directional flow rate of nL min−1 was produced. This cell micropump system could be further developed as a self-actuated and efficient mechanochemical transducer requiring no external energy sources for various purposes in the future.
Introduction
Integration of various chemical devices and complex operations onto a microchip, often referred to as micro-total analysis systems (µ-TAS) or labs-on-a-chip, is advancing very rapidly.1–3 New concepts in integrated miniaturized chemistry aims to create both a new academic field and a new associated (bio)chemical industry exploiting advantages of micron dimensions.4–6 As living cellular systems often exhibit complex compartmentalized reaction sequences and unique reagents (e.g., enzymes and coupled processes), harnessing cell-based reactions by incorporating cells into µ-TAS systems is now frequently reported. Such cellular functions are practical and useful because the scale of the fluidic microvolume within a typical microchip is roughly proportional to cell sizes and processing capabilities. Some efficient bioreactors and bioassay systems using cellular functions have been produced.7–9 However, to date, only cellular biochemical functions have been used to enhance microchip functions. Here, we have proposed to utilize cellular mechanical functions to produce distinctly different, more complicated and more efficient biochemical processes within a microvolume. Our concept utilizes cultured cardiomyocytes as microactuators, using the cell's endogenous ability to transform chemical energy into mechanical energy.Our proposed use of the cardiomyocyte's pulsatile pumping phenotype is also interesting to the field of microelectromechanical systems (MEMS) due to this unique actuating behavior. By utilizing micro-fabrication techniques, MEMS can create numerous physical, chemical and microfluidic components.10–12 Unlike conventional MEMS devices such as micropumps and micromotors, cardiomyocytes are autonomously actuated, exhibiting their natural chemomechanical phenotype using glucose and oxygen as their only exogenous energy sources. Creation of a cardiomyocyte actuator should also lead to an automatic and autonomous microsystem.
As examples of biomaterial actuators, only a few reports of adenosine triphosphate (ATP) biomolecular motors13,14 serve as precedents. Although biomolecular motors offer interesting alternatives to conventional actuators, they typically generate only a few piconewtons per molecule.15 By contrast, cardiomyocytes can generate a few micronewtons of force per cell,16 sufficiently robust to drive microstructures and fluids in practical device designs. Furthermore, more substantial contractile forces can be produced by using contiguous multi-cellular cardiomyocyte tissue constructs as measured using flat microcantilevers recently.17 By combining cardiomyocytes or cardiomyocyte tissue with micro-fabrication techniques, a cardiomyocyte bio-microactuator can be realized.
We have demonstrated capabilities to drive fabricated microstructures made of hydrogel18,19 and PDMS,20 and verified the concept of bio-microactuation. We have also proposed the concept of a cardiomyocyte pump as a practical bio-microactuator.21 Some similar studies such as a microorganisms carrier to transport objects in microfluidic devices,22 a walking bio-micromechanical device powered by muscular tissue,23 and a swimming robot actuated by living muscle tissue24 were reported recently. Also, a mechanism for fluid pumping by cardiomyocytes was demonstrated.25 The concept of this report describing oscillations of a membrane by self-organized cardiomyocytes is near to ours. However, a system with fluid pumping function using cardiomyocytes has not yet been reported.
To actively and reliably drive fluids, a strong contractile force with accompanying large volume change (i.e., pump stroke) is essential. To improve mechanical transduction and fluid dynamic performance, we now utilize a previously reported cultured cardiomyocyte sheet shown to exhibit cooperative contractile forces produced by collectively synchronous, pulsatile properties across larger-scale dimensions (i.e., cm2).26,27 Cardiomyocyte sheets are obtained routinely utilizing a commercial cell culture system based on the temperature-responsive polymer, poly(N-isopropylacrylamide) (PIPAAm). Importantly, these sheets can be harvested intact, handled, manipulated and transferred to various devices while maintaining their regular and robust pulsating phenotype.
In this report, we demonstrate fluid pumping using a cardiomyocyte sheet. First, control of fluid motion in a model microchannel using cardiomyocyte sheets without check valves is demonstrated to evaluate the potential performance of the prototype bio-microactuator. We describe fabrication processes for this actuator, introduce mild cardiomyocyte manipulation methods and measure how actuator performance responds to temperature change. Second, cell-induced directional fluid pumping within microchannels using microfabricated check valves is demonstrated.
Fig. 1 shows the design and the working principle of an actuated pump on chip using an on-board pulsatile cardiomyocyte sheet as the fluid pumping system. The device is covered with aqueous cell culture medium to supply cardiomyocytes with their energy source (glucose and oxygen). Fluid (medium) in the microchannel is driven by the collective, synchronous contracting forces of the cardiomyocyte sheet. A thin diaphragm and a push-bar structure are installed in the microchip in order to transmit the contractile forces of the square-shaped cardiomyocyte sheet to the fluid to produce oscillating microchannel flow. For directional flow, a check valve layer of stainless steel is placed between the chamber and microchannel layers, with inlet and outlet microchannels connected to the chamber.
 | ||
| Fig. 1 Design of a bio-actuated pump on a microchip powered by a cultured cardiomyocyte sheet. The microchip comprises five components i.e., the push-bar, diaphragm, chamber layer, microchannel layer, and check valve layer. The components except for the check valve layer are made of PDMS fabricated by replica molding. A cardiomyocyte sheet of contiguous pulsating cells attaches to the microchip surface pre-treated with cell adhesive protein, fibronectin. Diaphragm and fluid in the chamber are driven by the collective synchronized contractile motions of the attached cardiomyocyte sheet. By using micro-check valves, directional fluid flow is produced. (Upper) Schematic view. (Lower) Cross-sectional view along line A–B. | ||
Materials and methods
Fabrication of a microchip
Microchip components except for the check valve layer were fabricated using the replica molding method:28 templates were fabricated by standard photolithography patterning of photoresist. The microchip except for the check valve layer consists of four components: the push-bar, chamber layer, diaphragm membrane, and microchannel layer. These components were made of PDMS elastomer molded from the master templates.The master templates were fabricated by standard photolithography using photoresist (SU-8 3000, Kayaku Microchem) according to the manufacturer's data sheets. Briefly, a silicon wafer was (1) spin-coated with a 200–500 µm layer of SU-8; (2) placed on a hot plate to evaporate the solvent; (3) cooled at room temperature to prevent sticking of photoresist to the mask; (4) exposed to collimated UV light through a high-resolution transparency OHP film mask; (5) baked on a hot plate at 65 °C to cross-link the SU-8 resin, ramped up to 95 °C to accelerate this reaction; (6) developed in SU-8 Developer (Kayaku Microchem) to remove unexposed areas of photoresist; and (7) dried with a stream of nitrogen. Finally, the master was exposed to the vapors of surface modifier (Novec EGC-1700, Sumitomo 3M, Japan) for 1 min to prevent adhesion of PDMS to the master template during elastomer molding. The resulting surfaces were washed with deionized water and dried under a stream of nitrogen.29
Microchip components of PDMS curable elastomer were then molded from the master template. PDMS prepolymer (Sylgard 184 kit, Dow Corning) was (1) prepared by mixing PDMS base with a curing agent in a 10 ∶ 1 ratio by weight; (2) degassed under mild vacuum until the bubbles generated during stirring disappeared for the complete curing of PDMS; (3) poured over the micro-fabricated silicon wafer; (4) degassed again after pouring onto the master under mild vacuum; (5) cured at 120 °C for 1 hour; and (6) peeled from the template to produce the replica.29
Finally, a microchip was assembled from the different PDMS components. A diaphragm membrane was first attached to the chamber layer. A silicon wafer was (1) spin-coated with a 10 µm layer of prepared PDMS prepolymer; (2) attached to the fabricated chamber layer; (3) heated in an oven at 120 °C for 1 hour to harden the spin-coated PDMS membrane; and (4) peeled off with the chamber layer in 2-propanol using a pair of tweezers. The microchip components were then aligned under a microscope and stacked into the desired spatial configuration.
Preparation of neonatal rat cardiomyocytes
Primary neonatal rat cardiomyocytes were prepared according to previously published procedures.30 Briefly, ventricles from 0-day-old Wistar rats (Nisseizai) were digested at 37 °C in Hanks's solution containing collagenase (class II, Worthington Biochemical). Isolated cells were suspended in the culture medium consisting of 6% FBS, 40% Medium 199 (Gibco BRL), 0.2% penicillin-streptomycin solution, 2.7 mM glucose, and 54% balanced salt solution containing 116 mM NaCl, 1.0 mM NaH2PO4, 1.18 mM KCl, 0.87 mM CaCl2, and 26.2 mM NaHCO3. The primary cell suspension was prepared at a cell density of 2 × 106 ml−1.Preparation of square-shaped cardiomyocyte sheets
Cardiomyocyte sheets were fabricated from primary cell suspensions using polystyrene culture dishes grafted with a thin thermo-responsive polymer (poly(N-isopropylacrylamide), PIPAAM) overlayer that facilitates cell harvesting without destructive enzymes (e.g., no trypsin or dispase required).31–35 Square-shaped PIPAAm-grafted cell culture dishes were prepared by the following procedure.36 Tissue culture polystyrene (TCPS) dishes were (1) spread with N-isopropylacrylamide (IPAAm) monomer (kindly provided by Kohjin, Japan) in 2-propanol solution at a concentration of 55 wt% (70 µL); (2) subjected to electron beam irradiation using an area electron beam processing system, resulting in IPAAm polymerization and covalent bonding of IPAAm to the dish surface; and (3) rinsed with cold distilled water to remove ungrafted IPAAm, and dried in nitrogen gas. Next, the PIPAAm-grafted surface was (4) masked with a square glass cover slip (24 mm × 24 mm, Matsunami, Japan); (5) spread with N,N′-dimethylacrylamide (DMAAm) monomer (Wako Pure Chemicals, Japan) in 2-propanol solution at a concentration of 30 wt% (70 µL); (6) irradiated again with the electron beam instrument and washed. As a result, the center square area (24 mm × 24 mm) of each dish (60 mm diameter) was PIPAAm-grafted (temperature-responsive) and the surrounding area on its border was poly-DMAAm-grafted (non-cell adhesive). The dishes were gas-sterilized with ethylene oxide before cell culture use.Primary neonatal rat cardiomyocytes were then prepared and seeded at a cell density of 8 × 106 dish−1 and incubated at 37 °C in a humidified atmosphere with 5% CO2. Cardiomyocytes required 4 days on thermo-responsive cell culture surfaces to reach confluence, at which point, they pulsed visibly, synchronously and spontaneously in culture. Grafted PIPAAm surfaces are slightly hydrophobic and conducive to cell adhesion under culture conditions at 37 °C, but when reduced in culture to 32 °C, these surfaces undergo a spontaneous thermo-reversible change to hydrophilic and non–cell adhesive caused by rapid PIPAAm hydration and swelling.31–35 This unique surface transition facilitates successful culture to confluence, with maintenance of the cardiomyocyte phenotype, and then gently, spontaneous cell detachment from the grafted culture surfaces simply by reducing culture temperature. Cell detachment occurs without damage to the cardiomyocytes, and because it occurs without destructive enzymes, as a contiguous viable cell sheet.26,27,33 To release confluent cardiomyocytes as a cardiomyocyte sheet, the culture dish was incubated in another CO2 incubator at 20 °C. When culture temperature decreased from 37 °C to 20 °C, cardiomyocytes detached as a contiguous square cell sheet from the PIPAAm pattern within one hour and floated on the medium surface. The released cardiomyocyte sheet shrank significantly due to cytoskeletal tensile reorganization and its area contracted to 1 cm × 1 cm.26
Cardiomyocyte sheet transplantation onto a PDMS microchip
The assembled PDMS microchip was washed with ethanol, placed in a cell culture dish (35 mm diameter), sterilized using UV light for 15 min and immersed for 1 h in 50 µg mL−1 fibronectin (from bovine serum, Sigma) solution in phosphate buffered saline (PBS) at 37 °C to promote cardiomyocyte attachment. Next, a cardiomyocyte sheet was removed intact from its PIPAAm culture dish as described above and the entire cardiomyocyte sheet with medium was gently aspirated directly into a micropipette and transferred onto the microchip push-bar assembly. After spreading the cell sheet out onto the microchip components, the medium was aspirated to facilitate cardiomyocyte sheet attachment directly to the fibronectin-coated push-bar. Fig. 2A shows the microchip with the cardiomyocyte sheet in place. Within 2 h, the transferred cell sheet, pulsating continuously and spontaneously, securely attached itself to the bar, and new medium was then added to re-immerse the microchip for further culture. | ||
| Fig. 2 Fabrication of a bio-actuated pump on a microchip powered by a cultured cardiomyocyte sheet. (A) An actual photo of the microchip without check valves after transplantation of a cardiomyocyte sheet manipulated into place using a micropipette. The attached cardiomyocyte sheet is square shaped (1 cm × 1 cm). The microchip is square shaped (2 cm × 2 cm). The top and bottom diameters of the push-bar are 4 and 2 mm, respectively. The chamber has a 3 mm diameter and is 500 µm deep. Depth and width of the microchannel are both 200 µm. (B) An actual photo of the microchip with check valves after transplantation of a cardiomyocyte sheet. Check valves are installed in the inlet and outlet microchannels connected to the chamber. The main dimensions of the microchip are identical to that in Fig. (A). (C) A cantilever-type micro-check valve. The valve is made of polyimide and the substrate is made of stainless steel. Thicknesses of the polyimide film and stainless steel are 25 and 100 µm, respectively. (Upper) A microscopic picture of the check valve. (Lower) Cross-sectional view along line A–B. | ||
Installation of micro-check valves
Fig. 2B shows a fabricated cardiomyocyte pump on a microchip installed with check valves. A cultured cardiomyocyte sheet was attached to the push-bar by the same method mentioned above. A check valve layer made of stainless steel was placed between the chamber and microchannel layers, with inlet and outlet microchannels connected to the chamber as described in Fig. 1. To attach the valve layer to the PDMS chamber layer firmly and to avoid leakage of fluids from the chamber, the valve and PDMS chamber layers were glued with epoxy bonding agent (Araldite, Showa Highpolymer, Japan).The valve design is a cantilever-type reported previously,37 and was fabricated using a photosensitive polyimide (Fuchigami Micro, Japan). Briefly, the following procedure was used: (1) copper was plated and patterned on the surface of stainless steel (2 cm × 2 cm square, 100 µm thickness, Fuchigami Micro, Japan) (the copper formed a sacrificial layer making a gap between the stainless steel and a polyimide valve); (2) the photosensitive polyimide (25 µm thickness) was laminated and patterned over the copper on the stainless steel to form a rectangular cantilever-type check valve (700 µm × 800 µm); (3) the stainless steel was etched from the opposite side of the valve to make a hole for fluid transfer; (4) layers except for the polyimide were removed and a check valve was completed. Fig. 2C shows a microscopic image of a completed micro-check valve. In Fig. 2C(Upper), the dark part of the valve is a fixed piece attached to the stainless steel, and the light part of the valve is a movable part which was on the sacrificial copper layer before component etching and completion. A hole for fluidic transfer (200 µm diameter) is at the center of the movable part. Fluids flow directionally from the beneath to above the figure in Fig. 2C(Lower).
Results and discussion
Fluid bio-actuation on microchips without check valves
Spherical polystyrene tracking particles (Polybead, 1 µm diameter, Polysciences) were dispersed in cell culture media, and microchannel fluid behavior was observed directly using a phase contrast microscope (ELIPSE TE300, Nikon) with an objective lens (40×, 0.60-NA, 0.5 µm-lateral resolution) (Fig. 3A). The microscope was focused on the center of the microchannel and the image was recorded by an interfaced video cassette recorder (WV-DR9, Sony) through a CCD camera (HV-D28S, Nikon). Culture temperature was maintained at 37 °C during the observation using a thermo-plate (MATS-505R30, Tokai Hit). Before cardiomyocyte sheet transplantation, the tracking particles did not move except for Brownian motion. After cardiomyocyte sheet attachment, spontaneous, oscillating fluid motion in the microchannel produced from the repeated, regular pulsatile stroke movements of the cell sheet attached to the push-bar was observed at 37 °C, monitored by the movement of polystyrene tracking particles (see electronic supplementary information, Movie 1†). The displacement time-course trajectory for a select particle near the center of the channel (a particle inside an ellipse in Movie 1) as directly observed by video is plotted in Fig. 3B. Particle displacement to the right (x) was measured directly from sequential video frames of the microscopic video image every 0.033 s. Displacement at t = 0 s was defined as x = 0 µm. The fluid oscillating frequency was 0.7 Hz and the maximum linear displacement was 150 µm. This fluid oscillation continued for about one week in culture with medium changed every day. This is the first demonstration of cell-coupled fluid mechanical motion. | ||
| Fig. 3 Fluid actuation in a microchannel without check valves. (A) Schematic view describing the observation method and parameters to calculate performance of the cell-based fluid actuation on-chip. Fluid movement in the microchannel was directly visualized using polystyrene tracking particles and observed by a phase contrast microscope. Displacement (x) of one particle in the center of the microchannel was measured from video images. Displacement of the particle during relaxation (t = 0 s) was defined as x = 0 µm. Radius of the chamber: r0. Width of the microchannel: w. Depth of the microchannel: d. Displacement of one of the particles in the center of the microchannel: x. Volume change in the microchannel for one displacement: Δv. Volume change in the chamber for one displacement: ΔV. Displacement of the center of the diaphragm: z0. (B) Displacement time-course of one particle near the center of the channel (a particle inside an ellipse in Movie 1) for 5 s at 37 °C. Cardiomyocytes relax at t = 0 s. Cardiomyocytes contract to their maximum (x = 150 µm) at t = 0.2 s. | ||
In this rough estimation, we assumed that the stress on the membrane was uniform. For small displacements, z (the vertical displacement of a circular diaphragm where the distance from the center of the diaphragm is r) is approximated to the following equation:38
 | (1) |
 | (2) |
The relation between the volume change in the chamber (ΔV) and in the microchannel (Δv) is approximated as the following because the microchip is symmetry:
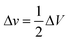 | (3) |
From the measured displacement of one of the particles near the center of the channel (x), Δv is estimated using the following approximation for volume change in a square geometry.
| Δv = 0.47 xwd | (4) |
| Q = fΔV | (5) |
Here, the estimated parameters are summarized for the ideal check valve bio-actuated device driven by the cardiomyocyte cell sheet: ΔV = 5.6 nL; z0 = 2.4 µm; Q = 0.24 µL min−1.
 | ||
| Fig. 4 Temperature response of cell-based fluid actuation upon increasing medium temperature on-chip from 30 °C to 40 °C. The synchronous pulsatile cell frequency and maximum displacement of one polystyrene particle in the center of the microchannel were measured for each temperature. Black plots indicate beat frequency and white plots indicate particle displacement. As culture temperature increased, cell pulsatile frequency increased while maximum particle displacement was slightly reduced. | ||
 | ||
| Fig. 5 Directional fluid pumping on-chip powered by a synchronously contracting cardiomyocyte sheet placed on the push-bar within a microchannel. (A) Schematic view describing the observation method. Fluid movement in the microchannel was directly visualized using fluorescent polystyrene tracking particles and observed by a fluorescent microscope. Displacements (x) of tracking particles at each center of the inlet and outlet microchannels were measured from video images. Displacements of each particle at t = 0 s were defined as x = 0 µm. (B) Displacement time-course of one particle near the center of the inlet microchannel (a particle inside an ellipse in Movie 2) for 20 s at 37 °C. (C) Displacement time-course of one particle near the center of the outlet microchannel (a particle inside an ellipse in Movie 3) for 20 s at 37 °C. | ||
Conclusions
A prototype pump on a fabricated elastomeric microchip powered by cardiomyocyte tissue has been demonstrated as a model bio-microactuator. The device comprised a simple PDMS microchip with a microchannel equipped with a diaphragm and a push-bar structure that could exploit collective cell forces integrated with a pulsating cardiomyocyte sheet. Fluid motion in a microchannel connected to a diaphragm chamber was demonstrated without check valves. Additionally, the temperature responses of the cell pulsatile frequency and force production were measured. When culture temperature increased, cell contraction frequency remained synchronous but increased, while stroke displacement was slightly reduced. This result suggests the possibility to control bio-microactuator performance using culture temperature. Finally, directional fluid pumping using fabricated check valves on-chip produced directional flow rates of about 2 nL min−1.This miniature bio-microactuator based on intrinsically pulsating cardiomyocyte tissue works with only chemical energy input to produce reliable mechanical force output. Therefore, that would be applicable to various microfluidic components used where electricity cannot be supplied. In the future, the demonstrated capabilities should enable fundamental changes in the concept of actuators, device miniaturization and cell-hybridized instrumentation.
Acknowledgements
The present work was partly supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan No. 16760215, 16651081, 17040026 and Grants-in-Aid for Scientific Research of the Japan Society for the Promotion of Science and the Promotion and Mutual Aid Corporation for Private School of Japan and K.M was supported by the Kurata Memorial Hitachi Science and Technology Foundation. We thank A. Hibara (University of Tokyo, Japan) and D. W. Grainger (Colorado State University, USA) for their critique and review of the manuscript.References
- D. J. Harrison, K. Fluri, K. Seiler, Z. H. Fan, C. S. Effenhauser and A. Manz, Science, 1993, 261, 895 CrossRef CAS.
- D. R. Reyes, D. Iossiidis, P.-A. Auroux and A. Manz, Anal. Chem., 2002, 74, 2623 CrossRef CAS.
- P.-A. Auroux, D. Iossiidis, D. R. Reyes and A. Manz, Anal. Chem., 2002, 74, 2637 CrossRef CAS.
- D. J. Beebe, J. S. Moore, J. M. Bauer, Q. Yu, R. H. Liu, C. Devadoss and B.-H. Jo, Nature, 2000, 404, 588 CrossRef CAS.
- B. Zhao, J. S. Moore and D. J. Beebe, Science, 2001, 291, 1023 CrossRef CAS.
- J. Kobayashi, Y. Mori, K. Okamoto, R. Akiyama, M. Ueno, T. Kitamori and S. Kobayashi, Science, 2004, 304, 1305 CrossRef CAS.
- S. Takayama, J. C. Mcdonald, E. Ostuni, M. N. Liang, P. J. A. Kenis, R. F. Ismagilov and G. M. Whitesides, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 5545 CrossRef CAS.
- W. Gu, X. Zhu, N. Futai, B. S. Cho and S. Takayama, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 15861 CrossRef CAS.
- M. Goto, K. Sato, A. Murakami, M. Tokeshi and T. Kitamori, Anal. Chem., 2005, 77, 2125 CrossRef CAS.
- T. Thorsen, S. J. Maerkl and S. R. Quake, Science, 2002, 298, 580 CrossRef CAS.
- A. Groisman, M. Enzelberger and S. R. Quake, Science, 2003, 300, 955 CrossRef CAS.
- D. J. Laser and J. G. Santiago, J. Micromech. Microeng., 2004, 14, R35 CrossRef.
- R. K. Soong, G. D. Bachand, H. P. Neves, A. G. Olkhovets, H. G. Craighead and C. D. Montemagno, Science, 2000, 290, 1555 CrossRef CAS.
- Y. Rondelez, G. Tresset, T. Nakashima, Y. Kato-Yamada, H. Fujita, S. Takeuchi and H. Noji, Nature, 2005, 433, 773 CrossRef CAS.
- M. E. Fisher and A. B. Kolomeisky, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 6597 CrossRef CAS.
- S. Nishimura, S. Yasuda, M. Katoh, K. P. Yamada, H. Yamashita, Y. Saeki, K. Sunagawa, R. Nagai, T. Hisada and S. Sugiura, Am. J. Physiol., 2004, 287, H196 Search PubMed.
- J. Park, J. Ryu, S. K. Choi, E. Seo, J. M. Cha, S. Ryu, J. Kim, B. Kim and S. H. Lee, Anal. Chem., 2005, 77, 6571 CrossRef CAS.
- K. Morishima, Y. Tanaka, K. Sato, M. Ebara, T. Shimizu, M. Yamato, A. Kikuch, T. Okano and T. Kitamori, Micro Total Analysis Systems, 2003, ed. M. A. Northrup, K. F. Jensen and D. J. Harrison, the Transducers Research Foundation, San Diego, 2003, vol. 2, p. 1125 Search PubMed.
- K. Morishima, Y. Tanaka, M. Ebara, T. Shimizu, M. Yamato, A. Kikuchi, T. Okano and T. Kitamori, Sens. Actuators, B DOI:10.1016/j.snb.2005.11.063.
- Y. Tanaka, K. Morishima, T. Shimizu, A. Kikuchi, M. Yamato, T. Okano and T. Kitamori, Lab Chip 10.1039/b512099c.
- Y. Tanaka, K. Morishima, T. Shimizu, A. Kikuchi, M. Yamato, T. Okano and T. Kitamori, Micro Total Analysis Systems, 2004, ed. T. Laurell, J. Nilsson, K. F. Jensen, D. J. Harrison and J. P. Kutter, the Royal Society of Chemistry, Cambridge, 2004, vol. 1, p. 378 Search PubMed.
- D. B. Weibel, P. Garstecki, D. Ryan, W. R. DiLuzio, M. Mayer, J. E. Seto and G. M. Whitesides, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11963 CrossRef CAS.
- J. Xi, J. J. Schmidt and C. D. Montemagno, Nat. Mater., 2005, 4, 180 CrossRef CAS.
- H. Herr and R. G. Dennis, J. Neuroeng. Rehabil., 2004, 1, 6 Search PubMed.
- J. Park, C. Kim Il, J. Cha, J. Lee and B. Kim, Micro Total Analysis Systems, 2005, ed. K. F. Jensen, J. Han, D. J. Harrison and J. Voldman, the Transducers Research Foundation, San Diego, 2005, vol. 1, p. 587 Search PubMed.
- T. Shimizu, M. Yamato, Y. Isoi, T. Akutsu, T. Setomaru, K. Abe, A. Kikuchi, M. Umezu and T. Okano, Circ. Res., 2002, 90, e40 Search PubMed.
- T. Shimizu, M. Yamato, A. Kikuchi and T. Okano, Biomaterials, 2003, 24, 2309 CrossRef CAS.
- Y. N. Xia and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 550 CrossRef CAS.
- A. Tourovskaia, T. Barber, B. T. Wickes, D. Hirdes, B. Grin, D. G. Castner, K. E. Healy and A. Folch, Langmuir, 2003, 19, 4754 CrossRef CAS.
- K. Kinugawa, T. Shimizu, A. Yao, O. Kohmoto, T. Serizawa and T. Takahashi, Circ. Res., 1997, 81, 911 Search PubMed.
- A. Kushida, M. Yamato, C. Konno, A. Kikuchi, Y. Sakurai and T. Okano, J. Biomed. Mater. Res., 1999, 45, 355 CrossRef CAS.
- M. Yamato, C. Konno, M. Utsumi, A. Kikuchi and T. Okano, Biomaterials, 2002, 23, 561 CrossRef.
- T. Shimizu, M. Yamato, T. Akutsu, T. Shibata, Y. Isoi, A. Kikuchi, M. Umezu and T. Okano, J. Biomed. Mater. Res., 2002, 60, 110 CrossRef CAS.
- Y. Akiyama, A. Kikuchi, M. Yamato and T. Okano, Langmuir, 2004, 20, 5506 CrossRef CAS.
- Y. Tsuda, A. Kikuchi, M. Yamato, A. Nakao, Y. Sakurai, M. Umezu and T. Okano, Biomaterials, 2005, 26, 1885 CrossRef CAS.
- M. Hirose, O. H. Kwon, M. Yamato, A. Kikuchi and T. Okano, Biomacromol., 2000, 1, 377 Search PubMed.
- N. Honda, S. Shoji, M. Isomura, Y. Ashihara, K. Akahori and H. Sato, Micro Total Analysis Systems, 2001, ed. J. M. Ramsey and A. van den Berg, Kluwer Academic Publishers, Dordrecht, 2001, p. 403 Search PubMed.
- C. T. Loy, S. C. Pradhan, T. Y. Ng and K. Y. Lam, J. Micromech. Microeng., 1999, 9, 341 CrossRef.
- K. Sato, M. Tokeshi, T. Odake, H. Kimura, T. Ooi, M. Nakao and T. Kitamori, Anal. Chem., 2000, 72, 1144 CrossRef CAS.
- H. Hisamoto, Y. Shimizu, K. Uchiyama, M. Tokeshi, Y. Kikutani, A. Hibara and T. Kitamori, Anal. Chem., 2003, 75, 350 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Movie 1: Real-time fluid flow in the microchannel (200 µm depth and width) by a pulsating cardiomyocyte sheet without check valves. Flow is visualized in situ using polystyrene tracking particles under microscopy. An ellipse encloses a polystyrene tracking particle. Movie 2: Real-time fluid flow produced by a pulsating cardiomyocyte sheet in the inlet microchannel with check valves. Flow is visualized in situ using fluorescent polystyrene tracking particles under fluorescent microscopy. An ellipse encloses a polystyrene tracking particle. Movie 3: Real-time fluid flow produced by a pulsating cardiomyocyte sheet in the outlet microchannel with check valves. An ellipse encloses a polystyrene tracking particle. See DOI: 10.1039/b515149j |
| This journal is © The Royal Society of Chemistry 2006 |
