Selective intercalation of chlorophenoxyacetates into the layered double hydroxide [LiAl2(OH)6]Cl·xH2O†
Anusha
Ragavan
,
Aamir
Khan
and
Dermot
O'Hare
*
Chemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford, OX1 3TA, UK. E-mail: dermot.ohare@chem.ox.ac.uk
First published on 6th September 2006
Abstract
Chlorophenoxyacetic acids are a well known family of herbicides. 4-Chlorophenoxyacetate (4-CPA), 2,4-dichlorophenoxyacetic (2,4-D), 2,4,5-trichlorophenoxyacetate, (2,4,5-T) have been intercalated into [LiAl2(OH)6]Cl·xH2O LDH ([Li–Al–Cl] LDH) by ion-exchange. The kinetics and mechanisms involved in the intercalation of each chlorophenoxyacetate have been studied by time-resolved in-situ energy dispersive X-ray powder diffraction (EDXRD). The kinetic preferential order of the intercalation of these herbicides into [Li–Al–Cl] LDH is found to be 4-CPA > 2,4-D > 2,4,5-T. In contrast, thermodynamic guest preference, as determined by performing competitive intercalation reactions is 2,4-D > 4-CPA > 2,4,5-T. The results indicate chlorophenoxyacetate-LDH compounds could have application for the purification, storage and release of chlorophenoxyacetic acid–based agrochemicals.
Introduction
Molecular recognition plays a vital role in many chemical technologies including chemical sensing,1,2 catalysis,3,4 and separation science.5,6 Generally, molecular recognition describes the selective binding of a guest species to a complementary host material. However, in the case of crystalline solids it implies accessibility to sites of complementary size, shape, charge and chemical functionality. In most cases, intercalation reactions proceed by relatively unselective processes, as the interlayer space expands to accommodate the guest species resulting in little size or shape selectivity. However, the layered double hydroxide host materials (LDHs), represented by the general formula [M2+1–xM3+x(OH)2]p+(An−)p/n·mH2O (where M2+ is typically Mg, Zn, Cu, Ni and Co; M3+ = Al, Ga; A = organic or inorganic anion) exhibit an excellent selectivity for a range of organic anions.7–16 These studies have highlighted the potential application of LDHs in separation science. For example, we and others have recently briefly reported the intercalation and controlled release of 2,4 dichlorophenoxyacetic acid in an LDH.17,18In this paper, we describe a full study of the selective intercalation of a range of chlorophenoxyacetates (Fig. 1); 4-chlorophenoxyacetate (4-CPA), 2,4-dichlorophenoxyacetate (2,4-D) and 2,4,5-trichlorophenoxyacetate, (2,4,5-T) into [LiAl2(OH)6]Cl·xH2O LDH ([Li–Al–Cl] LDH). These chemicals are the most widely used herbicides, and are employed worldwide on a large scale to control weeds in fields, grasslands, lawns and recreation areas.19–21 These acids act by mimicing the principal plant growth hormone indoleacetic acid. Recently, 2,4,5-trichlorophenoxyacetic acid has been partially banned from use as an herbicide as it usually contains minute quantities of one of the most toxic substances known to man: 2,3,7,8-tetracholorodibenzo-p-dioxin (TCDD or dioxin)
 | ||
| Fig. 1 Molecular structures and abbreviations for chlorophenoxyacetate herbicides. | ||
Dichlorophenoxyacetic acid is industrially prepared by the chlorination of phenoxyacetic acid22 or by the condensation of 2,4-dichlorophenol with monochloroacetic acid.23 All possible chlorophenoxyacetic acids are produced as biproducts in considerable amounts in the former method, whereas they are formed in trace amounts in latter. It is very important to be able to analyse commercial formulations and environmental samples, for monitoring or studying their fate in water, soil and sediment matrices. However, these compounds are difficult to separate and analyse and a variety of elaborate GC- and HPLC-based methods have been developed.
We were interested to explore a possible new simple and cost effective method for the separation of these widely used herbicides, using selective intercalation into [Li–Al–Cl] LDH. Additionally, it is of interest to determine how the extent of chlorine substitution affects the selective intercalation. In this work, the kinetic and thermodynamic orders of preferential selectivity has been determined and compared.
Experimental
Synthesis
[Li–Al–Cl] LDH was prepared by stirring a suspension of 3 g of γ-Al(OH)3, (0.038 mol) in 30 ml deionised water with a four-fold molar excess of LiCl at 90 °C for 48 h. The resulting white powder was washed with water and acetone and dried under vacuum.All chlorophenoxy herbicides were purchased in their acid forms from Aldrich. Solutions of the anionic salts were prepared by stirring the acids with one molar equivalent of NEt3 in 10 ml of a water–ethanol (50:50 v/v) mixture.
Characterisation
| α(t) = Ihkl(t)/Ihkl(max) | (1) |
| α = 1 − exp[−(kt)n] | (2) |
This equation is found to be most applicable in the range 0.15 < α < 0.8. k is the rate constant of the reaction and the exponent n can be used to predict the mechanism of intercalation.
The values of k and n can be easily obtained using a Sharp–Hancock plot, based on eqn 3.27
| ln[–ln(1 − α)] = nln(t) + nlnk | (3) |
Result and discussion
Rates of intercalation: kinetic preferences
The rate of interaction of 4-CPA, 2,4-D, and 2,4,5-T in [Li–Al–Cl] LDH was determined using time-resolved in-situ EDXRD experiments. Using in-situ EDXRD we were able to monitor the decrease in intensity of the Bragg reflections due to the host lattice and the increase in the intensity of the new Bragg reflections due to the herbicide intercalated LDH. It was necessary to carry individual reactions with each herbicides with the LDH host as the basal spacings of each of the herbicide intercalates were so similar that individual Bragg reflections from each new phase could not be resolved.The EDXRD data show that intercalation of 4-CPA, 2,4-D, or 2,4,5-T in [Li–Al–Cl] LDH process proceeding directly from the host to the final product; no crystalline intermediate phases were observed. In the case of 4-CPA the intercalation reaction was found to be very rapid, even using 10–30 s data acquisition times we could only observe the reaction using in-situ EDXRD at 0 and 5 °C. The intercalation of 2,4-D and 2,4,5-T into [Li–Al–Cl] LDH were studied over the temperature ranges 50–70 °C and 50–80 °C respectively. The extent of reaction (α) vs. time plots obtained for the 2,4,5-T intercalation reactions are illustrated in Fig. 2.
![Least squares fits of the Avrami-Erofe'ev equation to the data collected for the intercalation of 2,4,5-T into [Li–Al–Cl] LDH at 50 °C (■), 60 °C (●) 70 °C (▲), and 80 °C (▼).](/image/article/2006/JM/b610766d/b610766d-f2.gif) | ||
| Fig. 2 Least squares fits of the Avrami-Erofe'ev equation to the data collected for the intercalation of 2,4,5-T into [Li–Al–Cl] LDH at 50 °C (■), 60 °C (●) 70 °C (▲), and 80 °C (▼). | ||
The values of n obtained from the least squares fits of the αvs. time data to the Avrami-Erofe'ev expression (eqn 2) for the intercalation of 2,4,5-T were found to be in the range 1.58–1.85. Theoretically this suggests a mechanism involving two dimensional phase boundary control following deceleratory nucleation. The values of n for the intercalation of 2,4-D fall in the range 0.99–1.29, suggesting the mechanism of two-dimensional diffusion control following instantaneous nucleation. In contrast, values of n between 0.5 and 0.7 indicate a mechanism of pure diffusion control for the intercalation of 4-CPA. Sharp–Hancock plots for the intercalation of 2,4,5-T are displayed in Fig. 3. The kinetic parameters obtained from the Sharp–Hancock plots are summarised in Table 1.
![Sharp–Hancock plots for the intercalation of 2,4,5-T into [Li–Al–Cl] LDH at 50 °C (■), 60 °C (●), 70 °C (▲) and 80 °C (▼).](/image/article/2006/JM/b610766d/b610766d-f3.gif) | ||
| Fig. 3 Sharp–Hancock plots for the intercalation of 2,4,5-T into [Li–Al–Cl] LDH at 50 °C (■), 60 °C (●), 70 °C (▲) and 80 °C (▼). | ||
| Guest | Temperature (°C) | n | k (×10−3/s−1) | t 0.5/s |
|---|---|---|---|---|
| 2,4,5-T | 50 | 1.24 ± 0.04 | 0.85 ± 0.03 | 952 |
| 60 | 1.47 ± 0.04 | 1.89 ± 0.09 | 456 | |
| 70 | 1.65 ± 0.07 | 2.76 ± 0.40 | 317 | |
| 80 | 1.86 ± 0.17 | 4.98 ± 1.68 | 165 | |
| 2,4-D | 50 | 1.48 ± 0.06 | 2.63 ± 0.35 | 295 |
| 55 | 1.38 ± 0.04 | 3.41 ± 0.95 | 214 | |
| 65 | 1.42 ± 0.11 | 5.24 ± 1.77 | 153 | |
| 70 | 1.11 ± 0.07 | 8.82 ± 2.30 | 87 | |
| 4-CPA | 0 | 0.75 ± 0.02 | 2.01 ± 0.43 | 343 |
| 5 | 0.49 ± 0.02 | 2.73 ± 0.97 | 197 |
Using the temperature dependence of the rate of intercalation of each chlorophenoxyacetate into [Li–Al–Cl] LDH the activation energies involved in these reactions may be determined from the Arrhenius equation. The activation energies for the intercalation of 4-CPA, 2,4-D, and 2,4,5-T were determined as 43, 53.6 ± 9.4 and 61.7 ± 9.1 kJ mol−1 respectively. Thus the kinetic preferences are in the order 4-CPA > 2,4-D > 2,4,5-T. This order correlates with the size of the guests; the highest activation energy corresponds to intercalation of the biggest guest ion, 2,4,5-T. The proposed reaction mechanism of two dimensional phase-boundary control following deceleratory nucleation for 2,4,5-T also implies that the rate of intercalation is limited by the rate at which the interlayer space expands to accommodate the guest. In the case of 4-CPA, the mechanism is believed to be one of pure diffusion control, where transport of guest to the host is the rate determining step, and the 4-CPA anions intercalate as soon as they reach the layer edges. This is consistent with the lowest activation being for the intercalation of 4-CPA. Two dimensional diffusion control following instantaneous nucleation was suggested as the mechanism for the intercalation of 2,4-D. This is intermediate between the mechanisms for 2,4,5-T and 4-CPA. Activation energies for diffusion controlled reactions are generally lower than for phase boundary controlled cases.
Thermodynamic preferences
The order of thermodynamic preference of intercalation of 4-CPA, 2,4-D and 2,4,5-T in [Li–Al–Cl] LDH was determined by performing competitive intercalation reactions using an equimolar mixture of the herbicides. The quantitative determination of the extent to which each anion was intercalated was determined by performing a secondary exchange reaction on the isolated intercalated LDH phases. The intercalated LDH phases were treated with a five-fold molar excess of Na2CO3 in D2O at 80 °C overnight to release all the herbicide ions, solution 1H NMR was then used to determine the ratios of each component. In order to try and deconvolute the enthalpic and entropic contributions these competitive intercalation reactions were also performed at different temperatures using different binary combinations of the three herbicides. The intercalation preferences are given in Fig. 4.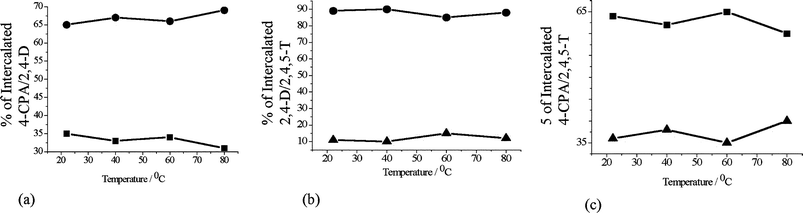 | ||
| Fig. 4 The percentage of each guest intercalated (as a function of temperature) in competitive intercalation reactions (a) 4-CPA (■) vs. 2,4-D (●); (b) 2,4-D (●) vs. 2,4,5-T (▲); (c) 4-CPA (■) vs. 2,4,5-T (▲). | ||
When equimolar amounts of 2,4-D and 4-CPA were stirred with [Li–Al–Cl] LDH 2,4-D was always preferentially intercalated in the host. The selectivity is found to be temperature independent, within experimental error and on average the LDH host was found to contain 65% 2,4-D and 35% 4-CPA. This is despite the fact that the rate of intercalation of pure 4-CPA in [Li–Al–Cl] is much faster than the rate of intercalation of 2,4-D. 2,4-D is always intercalated in preference to 2,4,5-T in the competitive intercalation of 2,4-D vs. 2,4,5-T. Again, the competitive interaction is not found to be temperature dependent. In this case the LDH was found to contain 88% 2,4-D and 12% 2,4,5-T. In the competitive intercalation of 4-CPA vs. 2,4,5-T, 4-CPA is found to intercalate in preference to 2,4,5-T. The intercalate contains ca. 63% of 4-CPA and ca. 37% of 2,4,5-T regardless of temperature. From these experiments, we can construct a preference order of 2,4-D > 4-CPA > 2,4,5-T. In order to confirm this order further reactions were performed with an equimolar solution of all three herbicides (a ternary mixture) over the same temperature range. The selectivity as determined by 1H NMR analysis is illustrated in Fig. 5.
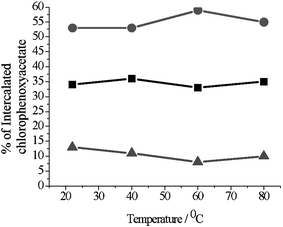 | ||
| Fig. 5 The percentages of 4-CPA (■) 2,4-D (●) and 2,4,5-T (▲) intercalated from a three components mixture as a function of temperature. | ||
![Schematic illustration of the probable interlayer arrangement of 2,4-D in [Li–Al–Cl] LDH.](/image/article/2006/JM/b610766d/b610766d-f6.gif) | ||
| Fig. 6 Schematic illustration of the probable interlayer arrangement of 2,4-D in [Li–Al–Cl] LDH. | ||
As expected, the same order of selectivity was obtained in this experiment. The selectivity of intercalation was again found to be temperature independent. On average, the LDH host was found to contain 53% 2,4-D, 35% 4-CPA and 12% 2,4,5-T. The synthesis of chlorophenoxyacetic acids involves the catalytic chlorination of phenol followed by condensation with a chloroacetate salt. Various impurities are formed in this process since the chlorination step is not site specific. Our results show that LDH materials could be used to purify chlorophenoxyacetic acids.
There are number of interlinked factors determining the selectivity of anion intercalation in LDH hosts. The principle factors include host–initial anion, host–final anion and anion–anion interactions in the interlayer region, and the solvation of the guest anions, (both the original guest anion in solution and the newly deintercalated anion). There are several potential interactions between the host and guests ions: the electrostatic interaction, H-bonding, and dipole–dipole interactions are particularly important. We have previously observed large temperature dependencies for preferential intercalation between isomeric compounds. However, in this case we find no temperature dedependence which suggests that the solvation entropies of the guests were not significant factors.
The dichloro derivative 2,4-D is thermodynamically preferred over the mono chloro derivative 4-CPA. We presume that the binding of the 2,4-D to the positively charged [LiAl2(OH)6]+ layers is stronger since both chloride substitutients can interact via hydrogen bonding in addition to the electrostatic interaction of the acetate group with the layers (Fig. 6). However, although 2,4,5-T has more chlorine substitutients than either 4-CPA and 2,4-D it is less favoured than the other two herbicides. For 2,4,5-T we would argue that there are likely to be more unfavourable Cl⋯Cl contacts in the interlayer region for this derivative compared to the other two derivatives.
Conclusions
The chlorophenoxyacetates (4-CPA, 2,4-D, 2,4,5-T) may be intercalated into [Li–Al–Cl] LDH via and ion-exchange reaction. The rate of intercalation of each of these guests was determined using time-resolved in-situ power diffraction experiments. The rates constants for each guest increased in the order 4-CPA > 2,4-D > 2,4,5-T. Using mixtures of chlorophenoxyacetates we were able to determine which guests were thermodynamically favoured over the other. We found that the order of the thermodynamic preferences was 2,4-D > 4-CPA > 2,4,5-T. Since 4-CPA is kinetically favoured but not thermodynamically favoured over 2,4-D we presume that 4-CPA rapidly intercalates in [Li–Al–Cl] LDH and then on a longer time scale these ions are replaced by 2,4-D. Our results suggest that chlorophenoxyacetate LDH materials could have potential applications for the purification, storage and release of chlorophenoxyacetic acid–based agrochemicals.Acknowledgements
The authors wish to thank the EPSRC and A.R. thanks the Felix Scholarship Committee for financial support. In addition, the CCLRC are thanked for access to Station 16.4 of the UK SRS at the Daresbury Laboratory.References
- T. Bein, K. Brown, C. G. Frye and J. C. Brinker, J. Am. Chem. Soc., 1989, 111, 7640 CrossRef CAS.
- Y. Yang and T. Bein, Chem. Mater., 1992, 4, 975 CrossRef CAS.
- M. J. Thomas, Sci. Am., 1992, 26, 3.
- P.-S. E. Dai, Catal. Today, 1995, 26, 3 CrossRef.
- P. B. Venuto, Microporous Mater., 1994, 2, 297 CrossRef CAS.
- I. P. Zaragoza, L. A. Garcia-Serano and R. Santamaria, J. Phys. Chem. B, 2005, 109, 705 CrossRef CAS.
- S. Miyata, Clays Clay Miner., 1975, 23, 369 CrossRef CAS.
- S. Miyata, Clays Clay Miner., 1980, 28, 50 CrossRef CAS.
- S. Miyata, Clays Clay Miner., 1983, 31, 305 CrossRef CAS.
- B. Lotsch, F. Millange, R. I. Walton and D. O'Hare, Solid State Sci., 2001, 3, 883 CrossRef CAS.
- A. M. Fogg, J. S. Dunn, S.-G. Shyu, D. R. Cary and D. O'Hare, Chem. Mater., 1998, 10, 351 CrossRef CAS.
- A. M. Fogg, V. M. Green, H. G. Harvey and D. O'Hare, Adv. Mater., 1999, 11, 1466 CrossRef CAS.
- F. Millange, R. I. Walton, L. Lei and D. O'Hare, Chem. Mater., 2000, 12, 1990 CrossRef CAS.
- L. Lei, F. Millange, R. I. Walton and D. O'Hare, J. Mater. Chem., 2000, 10, 1881 RSC.
- L. Lei, R. P. Vijayan and D. O'Hare, J. Mater. Chem., 2001, 11, 3276 RSC.
- L. Lei, A. I. Khan and D. O'Hare, J. Solid State Chem., 2005, 178, 3648 CrossRef CAS; G. R. Williams, T. G. Dunbar, A. J. Beer, A. M. Fogg and D. O'Hare, J. Mater. Chem., 2006, 16, 1231 RSC.
- A. Ragavan, A. I. Khan and D. O'Hare, J. Phys. Chem. Solids, 2006, 67, 983 CrossRef.
- M. Lakraimi, A. Legrouri, A. Barroug, A. De Roy and J. P. Besse, J. Mater. Chem., 2000, 10, 1007 RSC.
- B. Yaron, Z. Gerstl and W. F. Spencer, Advances in Soil Science, Vol 3, Springer, New York, 1985, p122 Search PubMed.
- C. Hammer and H. Tukey, Science, 1944, 100, 154. 27.
- C. McCourty, Nature, 1989, 340, 179–183.
- N. M. Melnikov, Res. Rev., 1971, 36, 165–166 Search PubMed.
- S. S. Que Hee and R. G. Sutherland, ( 1981), The Phenoxyalkanoic Herbicides, Vol. I. Chemistry, Analysis and Environmental Pollution, Boca Raton, CRC Press, Inc., 321 pp Search PubMed.
- S. M. Clark, J. Appl. Crystallogr., 1995, 28, 646 CrossRef CAS.
- K. A. Tarasov, V. P. Isupov and D. O'Hare, Inorg. Chem., 2003, 42, 1919 CrossRef CAS.
- D. O'Hare, J. S. O. Evans, A. Fogg and S. O'Brien, Polyhedron, 2000, 19, 297 CrossRef CAS.
- J. D. Hancock and J. H. Sharp, J. Am. Ceram. Soc., 1972, 55, 74 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The following supporting information is available; (1) complete data obtained from time-resolved in-situ EDXRD experiments (2) tables detailing the percentages of chlorophenoxy acetates intercalated into [Li–Al–Cl] LDH at different temperatures from two- and three-component mixtures. See DOI: 10.1039/b610766d |
| This journal is © The Royal Society of Chemistry 2006 |
