Preparation and optical properties of silver chalcogenide coated gold nanorods†
Mingzhao Liu and Philippe Guyot-Sionnest*
James Franck Institute, The Ellen and Melvin Gordon Center for Integrative Science, The University of Chicago, 929 East 57th Street, Chicago, Illinois 60637, USA. E-mail: pgs@uchicago.edu; Tel: +1 773 703 3463
First published on 20th July 2006
Abstract
A homogeneous layer of silver sulfide or selenide was coated onto gold nanorods in aqueous solution by exposing Au/Ag core/shell nanorods to S2− or Se2− in an oxidizing environment. The formation of the silver chalcogenide layers was confirmed by transmission electron microscopy (TEM) and selective area electron diffraction (SAED). The longitudinal plasmon resonance of the gold nanorods shifted to the red and was attenuated. The experimental spectra agree well with a simulation based on confocal ellipsoids in the quasi-static limit. The synthetic procedure was also employed to coat silver sulfide or selenide onto gold bipyramids. A red-shift of the longitudinal plasmon resonance was also seen, although the coating was not as homogeneous. These novel composite materials may present interesting nonlinear optical properties.
Introduction
The plasmon resonance of noble metal nanoparticles provides interesting optical properties in the visible and near-infrared region.1,2 At the plasmon resonance, the electromagnetic field in the vicinity of the nanoparticles is significantly disturbed, leading to a huge local field enhancement.2 For a system with optical nonlinearities, the local field enhancement yields higher nonlinear responses. In recent years, the ultrafast optical nonlinearities of metal colloids have been studied extensively.3 The observed nonlinearities are mainly due to the thermalization of the conduction electrons and phonons. Another way of introducing enhanced nonlinearities is to immerse the metal nanoparticles into a nonlinear medium. As the dielectric constant of the medium changes, the plasmon resonance shifts, as seen in Mie's theory.4 These nonlinearities may lead to optical bistability under certain conditions.5Recently, there has been significant interest in hybrid nanostructures with tunable optical properties.6,7 Some functionalized plasmonic systems, with magnetic (magnetite-Au) or exitonic properties (PbSe-Au), have also been reported.8 In this paper, we report a procedure to coat a thin layer of silver sulfide or silver selenide of controllable thickness over gold nanorods.
Experimental
Preparation procedures
Single crystalline gold nanorods with diameter ∼14 nm and aspect ratio 3–5 were synthesized by the seed-mediated method.9,10 A homogeneous thin layer (1–2 nm) of silver was then chemically plated onto the nanorod surfaces.11 First, 70 mg PVP (polyvinylpyrrolidone) was dissolved into 5 mL Au nanorod solution with λmax ∼ 700–850 nm. AgNO3 solution (1 mM; 0.5–1 mL) was then mixed with the Au nanorod solution, followed by 0.2 mL 0.1 M L-ascorbic acid. Finally, 0.1 M NaOH solution was added dropwise to the solution to adjust the pH to 8. At pH 8, Ag+ is mildly reduced by L-ascorbic acid and coated over the Au nanorod surfaces.In order to convert the silver layer to silver sulfide, an excess of 10 mM freshly prepared sodium sulfide aqueous solution was mixed with the Au/Ag core/shell nanorods. The colour of the solution changed from green to pink upon mixing. The mixture was stirred in a vessel open to air for a few hours towards the end of the reaction. The final solution was more or less brownish, depending on the thickness of the starting silver layer. It is important for the solution to be in contact with air, as seen in the balanced reaction:
| 4 Ag + 2 S2− + O2 + 2 H2O → 2 Ag2S + 4 OH− |
To make a silver selenide layer instead, the sodium sulfide solution was replaced by selenourea ((NH2)2CSe). Selenourea is quickly hydrolyzed and releases free Se2−in situ. A similar reaction as above then leads to the formation of silver selenide. The solution of the silver selenide coated gold nanorods is dark brownish.
Characterizations
The optical absorption spectra were taken by a Hewlett-Packard 8453 UV-Vis spectrometer. The spectra extended into the near IR region (1000–1200 nm) were measured by a Fourier transform IR spectrometer. The TEM and SAED images of the samples were acquired with an FEI Tecnai F30 microscope operating at 300 kV.Results and discussion
Both silver compounds are narrow band gap semiconductors (∼1 eV for silver sulfide12 and ∼0.15 eV for silver selenide13) with a large refractive index at optical frequencies. Therefore, the longitudinal plasmon resonance should red-shift once the silver chalcogenide layer is coated over the gold nanorods.14The formation and the uniformity of the silver chalcogenides layers were checked with the Transmission Electron Microscope (TEM). The gold nanorods shown here have diameter 14.0 ± 2.9 nm and aspect ratio 3.3 ± 0.7. The statistics were obtained by measuring about 100 gold nanorods from the TEM images. As seen in Fig. 1(b), a layer of ∼2 nm thickness attributed to silver was coated onto the surface of the gold nanorods. After the conversion to sulfide or selenide, its thickness became ∼3.5 nm, leaving the gold core unchanged (Fig. 1(c), (d)). The thickness of the silver sulfide or selenide layer is controlled by the thickness of the silver layer, i.e., a thinner silver layer gives a thinner silver chalcogenide layer.
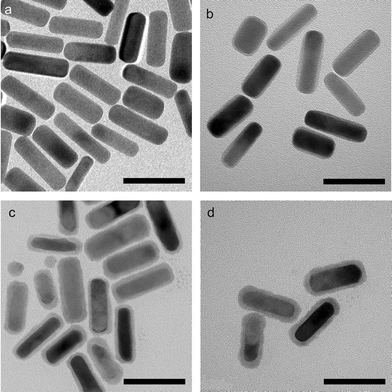 | ||
| Fig. 1 TEM images of (a) gold nanorods, (b) Au/Ag core/shell nanorods, (c) Au/Ag2S core/shell nanorods, and (d) Au/Ag2Se core/shell nanorods. Scale bars = 50 nm. | ||
In order to confirm the formation of the silver chalcogenide layers, we performed selective area electron diffraction (SAED) measurements on the samples (Fig. 2). The measured d values were then compared with the JCPDS (Joint Committee for Powder Diffraction Studies) cards. For the Au/Ag2S core/shell nanorods, the diffraction pattern is found to be consistent with monoclinic Ag2S (acanthite, PDF (Powder Diffraction File) card 75-1061), which is the low temperature phase (T < 450 K).15 For the Au/Ag2Se core/shell nanorods, the diffraction pattern is found to be consistent with orthorhombic Ag2Se (naumannite, PDF card 71–2410), which is also the low temperature phase (T < 400 K).7
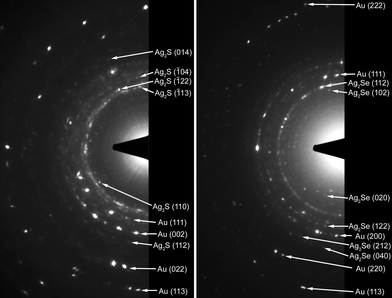 | ||
| Fig. 2 The SAED patterns from (left) Au/Ag2S core/shell nanorods, and (right) Au/Ag2Se core/shell nanorods. For each diffraction pattern, there are about 10 particles in the viewing area. Each arrow points to a characteristic spot in the diffraction ring. The diffractions from gold serve as the internal standard, with its lattice constant taken as 4.0786 Å.16 | ||
The UV-Vis-NIR spectra of the samples were taken to address the effect of the coating and they are shown in Fig. 3. The bare gold nanorods sample has the longitudinal plasmon resonance at 1.68 eV. The resonance is blue-shifted to 1.78 eV upon coating with 2 nm of silver.11 The intensity of the resonance is significantly enhanced, since more free electrons are involved into the plasmon. Once the silver layer is converted to silver chalcogenide, a red-shift of the longitudinal plasmon resonance is seen. The peak is shifted to 1.35 eV for the silver sulfide coating and to 1.28 eV for the silver selenide coating. A smaller red-shift is seen for thinner chalcogenide coatings. The final peak position also depends on the aspect ratio of the gold core. For gold nanorods with the plasmon resonance at 1.45 eV, a 3 nm silver selenide coating shifts the resonance to 1.1 eV. We note that the longitudinal plasmon resonance is attenuated as the silver layer is converted to silver sulfide or silver selenide. This can be explained by the high refractive indices of the silver chalcogenides, which screen the incident electromagnetic field.
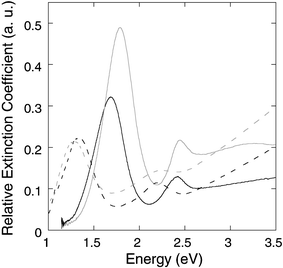 | ||
| Fig. 3 UV-Vis-NIR spectra of gold nanorods (solid black), Au/Ag core/shell nanorods (solid gray), Au/Ag2S core/shell nanorods (dashed black), and Au/Ag2Se core/shell nanorods (dashed gray). The absorbance values were normalized by the relative concentrations of each solution. | ||
The procedure described above should be applicable to other gold nanostructures. We tested it on gold bipyramids of ∼25 nm diameter and ∼80 nm length (Fig. 4(a)).10 Although a quite homogeneous silver layer can be coated onto gold nanorod surfaces, this is not the case for coating onto gold bipyramids. Instead, the coating is thicker on the facets, and much thinner around the corners, with almost no silver covering the tip at all (Fig. 4(b)). This coating eventually turns the overall shape of the nanoparticle from bipyramidal to ellipsoidal. We believe that silver preferentially coats the side facets of the bipyramids because of their highly-stepped structure.10 This allows the silver ions to be adsorbed and reduced on them preferably. Once the silver layer is converted to silver sulfide, the thickness profile is preserved, or even magnified (Fig. 4(c)). In contrast, the silver selenide layer appears to be quite even across the surface (Fig. 4(d)), implying that its formation might not be simply due to the diffusion of selenium atoms into the silver lattice.
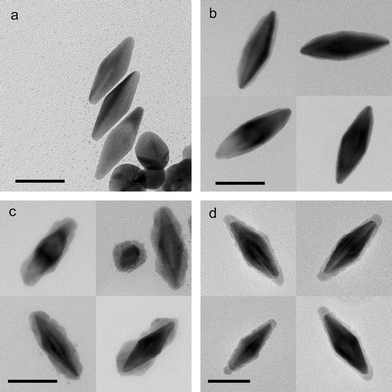 | ||
| Fig. 4 TEM images of (a) gold bipyramids, (b) with silver coating, (c) with silver sulfide coating, and (d) with silver selenide coating. Scale bars = 50 nm. | ||
The spectrum of the gold bipyramids is changed by the silver layer or the silver chalcogenide layer in a similar way as for the gold nanorods (Fig. 5). The pure Au sample has a very narrow longitudinal plasmon resonance at 1.56 eV, with a 0.12 eV linewidth. This is much narrower than that of the gold nanorods because of the excellent shape-monodispersivity of the gold bipyramids. The spectrum also shows pronounced extinction at 2–2.5 eV. While the extinction partly belongs to the transverse mode of the bipyramids, it is also due to the significant amount of spherical particles in the sample. The silver coating blue-shifts the longitudinal plasmon resonance to 1.76 eV, and broadens the peak to 0.14 eV, because of the extra dispersity on the shape, i.e., the thickness of the silver layer. Once the silver layer is converted to silver chalcogenides, the longitudinal plasmon resonance is red-shifted to 1.1–1.2 eV, and strongly attenuated.
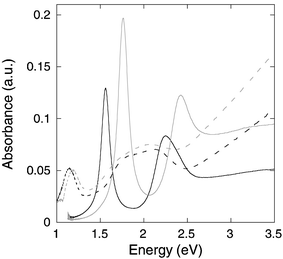 | ||
| Fig. 5 UV-Vis-NIR spectra of gold bipyramids (solid black), with silver coating (solid gray), with silver sulfide coating (dashed black), and with silver selenide coating (dashed gray). | ||
Numerical simulation of the optical spectra
A better understanding of the experimental spectra is obtained by simulating the spectra. In the model used, the gold core is considered to be a prolate spheroid with semiaxes a1 = b1 < c1 and dielectric function ε1(λ), and the shell is considered to be a spheroid shell confocal to the core with semiaxes a2 = b2 < c2 and dielectric function ε2(λ). The confocal condition requires c22 − a22 = c12 − a12. The rod is immersed in a medium (εm) with random orientation. In this model, the polarization by a plane monochromatic wave is analytically soluble when the rod size is much smaller than the wavelength (quasi-static approximation).2 The polarizabilities αj (j =1, 2, 3) along the three principal axes are given by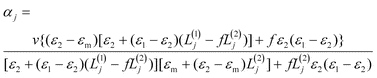 | (1) |
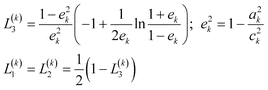 | (2) |
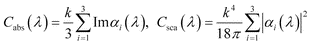 | (3) |
The simulation was made by averaging spectra from a Gaussian distribution of gold core aspect ratios, in order to address the inhomogeneous line-broadening in the experimental data. Bulk dielectric functions were used for the simulation.17 No simulation of silver selenide coated nanorods is provided, since the dielectric function reported for silver selenide does not cover the NIR range. For bare gold nanorods, the diameter is fixed at 14 nm, and a Gaussian distribution at 3.3 ± 0.7 is set for the aspect ratio, according to the measurement from the TEM images. The thicknesses of silver and silver sulfide layers at the equator are fixed at 2 nm and 3.5 nm, respectively. The simulation results for bare gold nanorods (Fig. 6) closely resemble the experimental spectrum in Fig. 3. For the silver coated ones, the longitudinal plasmon is enhanced and blue-shifted, in agreement with the experimental results. However, a smaller blue-shift is seen in the experimental spectrum. The reason is possibly that the thin layer (2 nm) of silver has a different dielectric constant than Ag bulk, as a result of the increased surface scattering of free electrons.11,18 For silver sulfide coated nanorods, the experimental data and simulation agree well within the whole spectral range. The longitudinal plasmon resonance is red-shifted by 0.3 eV and gets attenuated, although more attenuation is seen in the experimental result possibly because there is also a distribution of thicknesses of the silver sulfide layer, which is neglected in the simulation. The increasing extinction of Au/Ag2S core/shell nanorods in the UV range, which is a result of the increasing absorption by silver sulfide, is also nicely reproduced by the simulation.
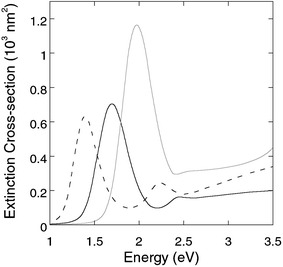 | ||
| Fig. 6 Simulated extinction spectra of gold nanorods (solid black), Au/Ag core/shell nanorods (solid gray), and Au/Ag2S core/shell nanorods (dashed black) immersed in a medium with εm = 1.77.19 | ||
Conclusions
In summary, this article reports a method to coat silver chalcogenide layer over gold nanorods. The layer of controlled thickness allows one to tune the plasmon resonance of gold nanoparticles, in good agreement with electromagnetic theory. Colloids of silver sulfide have also been reported to have excellent optical limiting properties due to its strong nonlinear optical absorption,20 which is commonly attributed to the photo-induced free carriers.21 The composite reported here, combining large field enhancement and large optical nonlinearity, provides an interesting avenue to enhanced nonlinear optical properties of nanomaterials.Acknowledgements
We thank Dr Xiaozhou Liao and Dr Joseph Pluth for their help with the measurement and interpretation of the SAED data. This work was supported by the University of Chicago MRSEC NSF-DMR under Grant DMR-0213745.Notes and references
- U. Kreibig and M. Vollmer, Optical properties of metal clusters, Springer, Berlin and New York, 1995 Search PubMed.
- C. F. Bohren and D. R. Huffman, Absorption and scattering of light by small particles, Wiley, New York, 1983 Search PubMed.
- C. Voisin, N. Del Fatti, D. Christofilos and F. Vallée, J. Phys. Chem. B, 2001, 105, 2264 CrossRef CAS; S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 8410 CrossRef CAS; M. Hu, X. Wang, G. V. Hartland, P. Mulvaney, J. P. Juste and J. E. Sader, J. Am. Chem. Soc., 2003, 125, 14925 CrossRef CAS; M. Pelton, M. Liu, S. Park, N. F. Scherer and P. Guyot-Sionnest, Phys. Rev. B, 2006, 73, 155419 CrossRef.
- G. Mie, Ann. Phys., 1908, 25, 377 Search PubMed.
- R. Neuendorf, M. Quinten and U. Kreibig, J. Chem. Phys., 1996, 104, 6348 CrossRef CAS.
- S. O. Obare, N. R. Jana and C. J. Murphy, Nano Lett., 2001, 1, 601 CrossRef CAS.
- U. Jeong and Y. Xia, Angew. Chem., Int. Ed., 2005, 44, 3099 CrossRef CAS.
- W. Shi, H. Zeng, Y. Sahoo, T. Y. Ohulchanskyy, Y. Ding, Z. L. Wang, M. Swihart and P. N. Prasad, Nano Lett., 2006, 6, 875 CrossRef CAS.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957 CrossRef CAS.
- M. Z. Liu and P. Guyot-Sionnest, J. Phys. Chem. B, 2005, 109, 22192 CrossRef CAS.
- M. Z. Liu and P. Guyot-Sionnest, J. Phys. Chem. B, 2004, 108, 5882 CrossRef CAS.
- J. M. Bennett, J. L. Stanford and E. J. Ashley, J. Opt. Soc. Am., 1969, 59, 499 Search PubMed.
- R. Dalven and R. Gill, Phys. Rev., 1967, 159, 645 CrossRef CAS.
- J. Pérez-Juste, I. Pastoriza-Santos, L. M. Liz-Marzán and P. Mulvaney, Coord. Chem. Rev., 2005, 249, 1870 CrossRef CAS.
- W. P. Lim, Z. Zhang, H. Y. Low and W. S. Chin, Angew. Chem., Int. Ed., 2004, 43, 5685 CrossRef CAS.
- CRC handbook of chemistry and physics, ed. D. R. Lide, 80th edn, CRC Press, Boca Raton, FL, 1999 Search PubMed.
- Dielectric functions of gold and silver for the simulations are taken from P. B. Johnson and R. W. Christy, Phys. Rev. B, 1972, 6, 4370 Search PubMed . Dielectric function of silver sulfide is taken from J. M. Bennett, J. L. Stanford and E. J. Ashley, J. Opt. Soc. Am., 1970, 60, 224 CrossRef CAS.
- A. Arbouet, C. Voisin, D. Christofilos, P. Langot, N. Del Fatti, F. Vallée, J. Lermé, G. Celep, E. Cottancin, M. Gaudry, M. Pellarin, M. Broyer, M. Maillard, M. P. Pileni and M. Treguer, Phys. Rev. Lett., 2003, 90, 177401 CrossRef CAS.
- εm = 1.77 refers to water at room temperature.
- Y.-P. Sun, J. E. Riggs, H. W. Rollins and R. Guduru, J. Phys. Chem. B, 1999, 103, 77 CrossRef CAS.
- M. Y. Han, W. Huang, C. H. Chew, L. M. Gan, X. J. Zhang and W. Ji, J. Phys. Chem. B, 1998, 102, 1884 CrossRef CAS.
Footnote |
| † This paper is part of a Journal of Materials Chemistry theme issue on Anisotropic Nanomaterials. Guest editor: Luis Liz-Marzan. |
| This journal is © The Royal Society of Chemistry 2006 |
