Polymeric materials for ophthalmic drug delivery: trends and perspectives
Eugen
Barbu
a,
Liliana
Verestiuc
b,
Thomas G.
Nevell
a and
John
Tsibouklis
*a
aSchool of Pharmacy and Biomedical Sciences, University of Portsmouth, St. Michael's Building, White Swan Road, Portsmouth PO1 2DT, UK. E-mail: john.tsibouklis@port.ac.uk; Fax: +44 (0) 23.92.84.3565; Tel: +44 (0) 23.92.84.3606
bFaculty of Medical Bioengineering, University of Medicine and Pharmacy “Gr.T.Popa”, 16 Universitatii St., Iasi, 6600, Romania
First published on 3rd July 2006
Abstract
Colloidal nanosystems based on biodegradable polymeric materials that combine the capabilities of stimulus response and molecular recognition promise significant improvements in the ocular delivery of therapeutic agents.
 Eugen Barbu | Eugen Barbu joined the staff at the University of Portsmouth in 2001 and is currently holding the position of Senior Research Fellow. He received his research training at the University of Ploiesti (Romania), the Organisch-Chemisches Institut der Universität Heidelberg (Germany), and the University of Portsmouth (UK—Leverhulme fellowship). He is currently involved the design and development of delivery systems aimed at mucosal surfaces, and use of responsive polymers in ophthalmic drug delivery. |
 Liliana Verestiuc | Liliana Verestiuc is a Senior Lecturer in Biomaterials at the University of Medicine and Pharmacy “Gr.T.Popa”, Iasi, Romania. She is interested in the applications of natural and synthetic polymers in biomedical sciences, and her current work focuses on use of smart polymers in drug delivery, and on tissue engineering and biocompatibility of implants. |
 Thomas G. Nevell | Thomas G. Nevell is currently occupying the position of Principal Lecturer. His research on the surface properties of materials started in 1987. Work on antifouling elastomers was followed by investigations of the surface properties and resistance to biofouling of non-toxic silicone elastomers and low-surface-energy fluoropolymers. |
 John Tsibouklis | John Tsibouklis is a Reader in Polymer Science. Current research efforts are directed towards many aspects of modern polymer science, including biomaterials and controlled drug delivery. Dr Tsibouklis has contributed to more than one hundred research publications, including five patents that are held by industry. |
Topical drug delivery to the eye is often impaired by removal mechanisms (blinking, tears) and by the barriers of the pre-corneal area. Conventional topical treatments have major drawbacks including poor ocular bioavailability (i.e. less than 5% of the administered active is absorbed or becomes available at the site of physiological activity), pulsed drug entry, systemic exposure, and, generally, a low effectiveness for intraocular drug delivery (Fig. 1).1,2 Therapeutic advances in this field are hindered by the difficulties of increasing bioavailability and of delivering actives to the site of action, especially in the treatment of the posterior of the eye.2,3 Very few new ophthalmic drug delivery systems have been commercialised so far, but current research on polymeric systems holds considerable promise. Certain polymers are already used as carriers or sustained release vehicles, while ‘smart’ hydrogels—that react to disease-specific environmental triggers and/or chemical signals to effect drug release—are emerging as components of a new generation of therapeutics.4,5
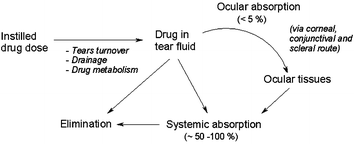 | ||
| Fig. 1 Absorption and dissipation pathway of a topically administered ophthalmic drug. | ||
Polymeric formulations for increasing bioavailability and sustaining release
An ideal polymeric drug carrier should have a loading capacity that can ensure therapeutic doses, be able to penetrate to—and/or reside at—the desired site of action, and, release the active in a controlled manner. It should also be non-toxic, biocompatible and biodegradable—the latter especially for intraocular administration. For most ophthalmic applications the carrier should not impede vision, since tolerability and acceptance by the patient are critical. The carrier's mucoadhesive properties are very important when surface eye diseases are targeted, while for the treatment of intraocular ailments it must be able to enhance corneal drug penetration.Polymeric vehicles for controlled drug release have been classified as: reservoir-type, where the polymer essentially coats the drug core, and matrix-type, where the active is homogeneously mixed with the polymer, or is bound to it through covalent or hydrogen/donor–acceptor interactions. The vehicles may assume a variety of forms, ranging from solutions or gels to colloidal systems or solid inserts. Polymeric components may be natural or synthetic, and their architecture (linear, grafted, branched, cross-linked, block, star-shaped, dendrimer etc)6 and hydrophilic/lypophilic balances are critical in determining the controlled release of the active.
Thiomers, which can form covalent disulfide bridges with cysteine-rich subdomains of mucins, have been examined as materials for mucoadhesive drug delivery: ocular inserts made of thiolated polyacrylic acids have shown promise in tests on human volunteers.7
Particulate systems coated with flexible, mucoadhesive polymer chains may be entrapped and/or bound in the mucus layer of the tear film or be retained in the conjunctival sac, thus avoiding drainage. High molecular weight (>100 kDa) electrically charged polymers are mucoadhesive, but this is not the only factor determining bioavailability.8
Other non-biodegradable bioadhesive materials for drug-release have been investigated, including: copolymers based on vinylpyrrolidone and/or (meth)acrylic acids;9,10 poly(dimethylsiloxane) and poly(N-isopropyl acrylamide) interpenetrating networks;11 and, poly(amidoamine) dendrimers.12 As a rule, increasing the crosslinking density of a polymeric network lowers diffusion rates, thereby improving the sustained release properties of the system. The network's mucoadhesive capability is reduced by crosslinking, however, thereby potentially cancelling any bioavailability benefit.
To effect sustained release (over several days) from hydrophilic hydrogel networks that are freely permeable to water, drugs must be associated strongly with the macromolecular chains. Poly(hydroxyethyl methacrylate) hydrogels loaded with dimyristoyl phosphatidylcholine (DMPC) liposomes are optically transparent and release drugs for a period of about 8 days.13 Since delivery rates from liposome-laden gels may be tailored by controlling particle size and drug loading, contact lenses can deliver therapeutic levels of drugs for several days.
Biocompatibility and biodegradability
Recent research has concentrated on biodegradable polymers, many of natural origin.14–18 Work on biodegradable crosslinkers that are commonly used for the construction of three-dimensional hydrogel networks has been reviewed.19 Materials based on poly(lactic) and poly(glycolic acid)s, or their copolymers and derivatives, have been formulated as implants,15 as micro/nanospheres and nanocapsules,16 and as films for glaucoma treatment.14 The in vitro release of methotrexate from poly(DL-lactide)-poly(ethylene glycol) diblock copolymer nanocapsules has been compared with that from poly(D,L-lactide) nanocapsules.17 Biodegradable poly(lactide-co-glycolide) microparticles have shown sustained retinal delivery of celecoxib and have inhibited diabetes-induced retinal oxidative damage.19 In these materials, the drug is released by bulk erosion of the matrix following the cleavage of the polymeric chains via autocatalytic acid/base and/or enzymatic hydrolysis; the products, lactic and glycolic acids, are metabolised to carbon dioxide and water. Comparative studies have shown a faster degradation of poly(lactic-co-glycolic acid) (PLGA) in vivo, suggesting a cell-mediated degradation process of the polymer, caused by giant cells and hydrolytic enzymes.20 Low molecular weight polymers tend to degrade rapidly; copolymers such as PLGA degrade faster than the corresponding homopolymers. These materials are particularly suitable for implantable devices or injectable micro(nano)spheres.2Poly(anhydride)s and poly(orthoester)s are promising materials, the controlled release properties of which are regulated mainly by surface degradation (rather than drug diffusion).21 Poly(anhydride)s are a unique class of polymers for drug delivery: some of them combine near zero-order drug release with biocompatibility and relatively rapid in vivo biodegradation. Further, the release rate from a polyanhydride-fabricated vehicle may be altered over a thousand fold by simple changes to the polymer backbone.22
Bioerodible poly(ortho esters)23 have shown excellent ocular biocompatibility and sustained controlled release of 5-fluorouracil, an antiproliferative agent used as an adjunct to glaucoma filtering surgery. Poly(ε-caprolactone) rod-shaped implants have been well tolerated by retinal tissue and are reported to release the corticosteroid triamcinolone acetonide over at least 4 weeks,24 but highly hydrophilic drugs are not compatible with these hydrophobic polymer-based carriers. By contrast, hydrophilic polymers of natural origin (chitosan, gellan, scleroglucan and hyaluronan) are non-toxic, biocompatible, biodegradable, and improve significantly the bioavailability of the active.25–27
Chitosan (essentially a cationic polysaccharide copolymer of 1,4-[2-amino-2-deoxy-β-D-glucopyranose] and 1,4-[2-acetamido-2-deoxy-β-D-glucopyranose]; Fig. 2a) has received particular attention.26,28 Sustained release has been achieved by grafting chitosan with poly(N-isopropyl acrylamide) to form a thermally responsive ophthalmic delivery vehicle,29 by formulation of chitosan as nanoparticles with Carbopol,30 and also in chitosan-coated niosomal timolol maleate formulations.31 Chitosan ocular implants are regarded as safe and are effective in terms of drug release.32 Results of confocal microscopy studies suggest that chitosan nanoparticles can penetrate into the corneal and conjunctival epithelia; a greater affinity of chitosan for some specific types of conjunctival cells has been suggested.33 Other modified polysaccharides, such as chondroitin 6-sulfate-graft-poloxamer copolymer, have been studied as transparent in situ gel-forming drug carriers.34
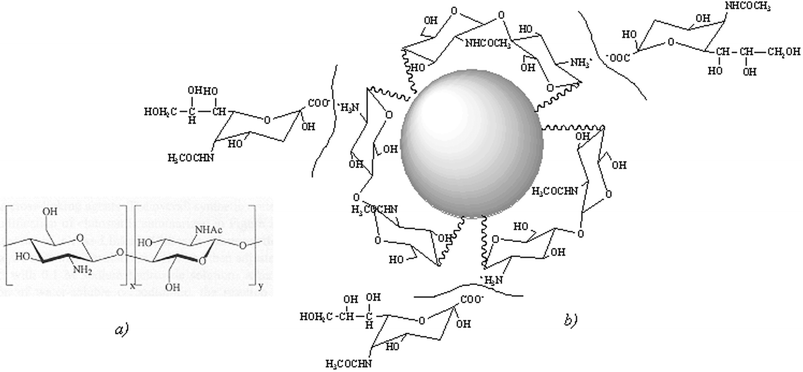 | ||
| Fig. 2 (a) Structure of chitosan: a biopolymer obtained hydrolytically from chitin of crustacean shells. (b) Interaction between chitosan-coated nanoparticles and sialic acid present in the mucin layer is expected to improve bioavailability. | ||
2-Hydroxypropyl-β-cyclodextrin has been used as a penetration enhancer in combination with chitosan35 and with a hydroxypropylmethylcellulose carrier of enoxacin;36 the applications of cyclodextrins in drug delivery have been reviewed recently.37–39
Nanoparticulate polymeric carriers
The formulation of biodegradable polymers as colloidal systems holds significant promise for ophthalmic drug delivery.40–44 A colloidal system is suitable for poorly water-soluble drugs, and would allow drop-wise administration while maintaining the drug activity at the site of action. Additionally, surface-modified nanoparticulate carriers may be used to accommodate a wide variety of actives (Fig. 3). Although several synthetic methods and drug loading techniques are reported to be safe and reproducible, no procedure for the formulation of drug-loaded nanoparticles has yet been standardised. The major developmental issues in the case of nanoparticles include formulation stability, particle size uniformity, control of drug release rate, and large-scale manufacture of sterile preparations.45 | ||
| Fig. 3 Lipophilic (a), ionic (b,c), and amphiphilic (d) nanoparticles. | ||
Nanosystems having surface-segregated chitosan or polyethyleneglycol have been found to be relatively stable and also efficient at overcoming mucosal barriers (Fig. 2b).46 Chitosan nanoparticles labelled with fluorescein isothiocyanate-modified bovine serum albumin have been shown to be well tolerated by the ocular surface tissues, and to be internalised by human conjunctival epithelial (IOBA-NHC) cells via an active transport mechanism that did not compromise cell viability.47 Thus, surface modification of colloidal drug vehicles might facilitate the modulation of carrier–ocular tissue interaction.
Perspectives
Considerable advances have been made towards the understanding of the structure and biochemistry of mucins.48 The exploitation of specific structural or chemical features for the development of drug delivery platforms represents a new area of research; advances in this area may lead to materials with selective affinity for the corneal tissue. Natural limiting factors, however, are the low concentration of mucins on the ocular tissue and the high mucin turnover rate.Dendrimers, which possess unique properties (multivalency, globular architecture and well-defined molecular weight) that make them promising new scaffolds for drug delivery,49–53 especially if formulated as micelles,44 may prove effective vehicles for ocular drug delivery.
Imprinted polymers are increasingly considered for biomedical applications, including drug delivery.52,54,55 Feedback-regulated drug delivery may be achievable by a combination of imprinted, stimuli-sensitive materials that would allow high drug-loading-capacity molecularly imprinted polymers (MIPs) to respond to external stimuli (slight changes in: pH, temperature, ionic strength, concentration of biomolecules; or, presence of specific receptors and/or ligands) and modulate the affinity of the network for the target molecules (bioactives), thus providing a regulatory capability for the release process (Fig. 4).
 | ||
| Fig. 4 Schematic of targeted drug delivery and intelligent active release with a molecularly imprinted carrier. | ||
As drugs can now be coupled to nanocarriers that are specific for cells and/or organs, nanotechnologies will play a vital role in the future of ophthalmic medication. Due to their ability to be easily transported in the sanguine system, to penetrate different tissues, and to carry therapeutic doses of drugs, polymeric nanomaterials offer the possibility to develop new diagnostic and therapeutic tools. For processing, the benefits of using supercritical fluids include more controllable particle size and high levels of diffusivity in both synthetic polymers and drugs.53,56 Copolymer vesicles and tubules43 and thermoresponsive polymeric micelles have been investigated as intelligent targeted carrier systems,39,42 whilst the versatility of polymers is turned to account in the design of new prodrugs;57 these approaches await validation in drug release ophthalmic applications, and most probably a combination of technologies holds the key to success.58
Progress in the characterisation of ocular enzyme systems and cellular transporters is likely to make transporter/receptor mediated ophthalmic drug delivery a viable approach.59,60 Finally, since drugs can be covalently coupled to the side chain of amino acids, making them recognizable by specific transporters, the broad substrate specificity and concentrative ability of amino acid transporters expressed in the eye may prove effective delivery systems for a wide variety of actives.61
References
- C. G. Wilson, Exp. Eye Res., 2004, 78, 737 CrossRef CAS.
- T. Yasukawa, Y. Ogura, E. Sakurai, Y. Tabata and H. Kimura, Adv. Drug Delivery Rev., 2005, 57, 2033 CrossRef CAS.
- P. M. Hughes, O. Olejnik, J. E. Chang-Lin and C. G. Wilson, Adv. Drug Delivery Rev., 2005, 57, 2010 CrossRef CAS.
- B. Twaites, C. D. Alarcon and C. Alexander, J. Mater. Chem., 2005, 15, 441 RSC.
- M. E. H. El-Sayed, A. S. Hoffman and P. S. Stayton, Expert Opin. Biol. Ther., 2005, 5, 23 Search PubMed.
- L. Y. Qiu and Y. H. Bae, Pharm. Res., 2006, 23, 1 CrossRef CAS.
- A. Bernkop-Schnurch, Adv. Drug Delivery Rev., 2005, 57, 1569 CrossRef.
- A. Ludwig, Adv. Drug Delivery Rev., 2005, 57, 1595 CrossRef CAS.
- E. Barbu, I. Sarvaiya, K. L. Green, T. G. Nevell and J. Tsibouklis, J. Biomed. Mater. Res., 2005, 74A, 598 CrossRef CAS.
- Y. Sultana, M. Aqil and A. Ali, Acta Pharm., 2005, 55, 305 Search PubMed.
- L. Liu and H. Sheardown, Biomaterials, 2005, 26, 233 CrossRef CAS.
- T. F. Vandamme and L. Brobeck, J. Controlled Release, 2005, 102, 23 CrossRef CAS.
- D. Gulsen and A. Chauhan, Invest. Ophthalmol. Visual Sci., 2004, 45, 2342 CrossRef.
- S. F. Huang, J. L. Chen, M. K. Yeh and C. H. Chiang, J. Ocul. Pharmacol. Ther., 2005, 21, 445 CrossRef CAS.
- S. L. Fialho and A. D. Cunha, Drug Delivery, 2005, 12, 109 CrossRef CAS.
- C. Andrieu-Soler, A. Aubert-Pouessel, M. Doat, S. Picaud, M. Halhal, M. Simonutti, M. C. Venier-Julienne, J. P. Benoit and F. Behar-Cohen, Molecular Vision, 2005, 11 Search PubMed.
- T. J. De Faria, A. M. De Campos and E. L. Senna, Macromol. Symp., 2005, 229, 228 CrossRef.
- S. P. Ayalasomayajula and U. B. Kompella, Eur. J. Pharmacol., 2005, 511, 191 CrossRef CAS.
- A. A. Thomas, I. T. Kim and P. F. Kiser, Tetrahedron Lett., 2005, 46, 8921 CrossRef CAS.
- S. H. Oh, S. G. Kang and J. H. Lee, J. Mater. Sci.: Mater. Med., 2006, 17, 131 CrossRef CAS; A. A. Van Apeldoorn, H. J. Van Manen, J. M. Bezemer, J. D. de Bruijin, C. A. Van Blitterswijk and C. Otto, J. Am. Chem. Soc., 2004, 126, 13226 CrossRef.
- C. S. Ha and J. A. Gardella, Chem. Rev., 2005, 105, 4205 CrossRef CAS.
- J. P. Jain, S. Modi, A. J. Domb and N. Kumar, J. Controlled Release, 2005, 103, 541 CrossRef CAS.
- J. Heller, Adv. Drug Delivery Rev., 2005, 57, 2053 CrossRef CAS.
- N. R. F. Beeley, J. V. Rossi, P. A. A. Mello, M. I. Mahmoud, G. Y. Fujii, E. De Juan and S. E. Varner, J. Biomed. Mater. Res., 2005, 73A, 437 CrossRef CAS.
- T. Coviello, A. Palleschi, M. Grassi, P. Matricardi, G. Bocchinfuso and F. Alhaique, Molecules, 2005, 10, 6 Search PubMed.
- M. Prabaharan and J. F. Mano, Drug Delivery, 2005, 12, 41 CrossRef CAS.
- Y.-H. Liao, S. A. Jones, B. Forbes, G. P. Martin and M. B. Brown, Drug Delivery, 2005, 12, 327 CrossRef CAS.
- M. Bodnar, J. F. Hartmann and J. Borbely, Biomacromolecules, 2005, 6, 2521 CrossRef CAS.
- Y. Cao, C. Zhang and Q. Ping, Polym. Mater. Sci. Eng., 2005, 21(6), 236 Search PubMed.
- H. J. Kao, H. R. Lin, Y. L. Lo and S. P. Yu, J. Pharm. Pharmacol., 2006, 58, 179 CrossRef.
- D. Aggarwal and I. P. Kaur, Int. J. Pharm., 2005, 209(1–2), 155 CrossRef.
- X.-D. You, Z.-Q. Jin, J.-G. Wu and Y.-P. Song, Ophthalmologica, 2005, 5, 74 Search PubMed.
- A. M. Campos, Y. Diebold, E. L. Carbalho, A. Sanchez and M. a. Jose Alonso, Pharm. Res., 2005, 22(6), 1007 CrossRef.
- M. K. Yoo, K. Y. Cho, H. H. Song and Y. J. Choi, Drug Dev. Ind. Pharm., 2005, 31, 455 CrossRef CAS.
- M. Prabaharan and J. F. Mano, Carbohydr. Polym., 2006, 63, 153 CrossRef CAS.
- Z. D. Liu, W. S. Pan, S. F. Nie, L. B. Zhang, X. G. Yang and J. W. Li, Drug Dev. Ind. Pharm., 2005, 31, 969 CrossRef CAS.
- S. Shimpi, B. Chauhan and P. Shimpi, Acta Pharm., 2005, 55, 139 Search PubMed.
- H. H. Sigurdsson, E. Stefansson, E. Gudmundsdottir, T. Eysteinsson, M. Thorsteinsdottir and T. Loftsson, J. Controlled Release, 2005, 102, 255 CrossRef CAS.
- S. V. Aathimanikandan, E. N. Savariar and S. Thayumanavan, J. Am. Chem. Soc., 2005, 127, 14922 CrossRef CAS.
- R. A. Bejjani, F. Behar-Cohen, D. Benezra, R. Gurny and F. Delie, Chimia, 2005, 59, 344 CrossRef.
- H. M. Aliabadi and A. Lavasanifar, Expert Opin. Drug Delivery, 2006, 3, 139 Search PubMed.
- M. Nakayama and T. Okano, Cancer Chemother., 2005, 32, 935 Search PubMed.
- I. W. Hamley, Soft Matter, 2005, 1, 36 RSC.
- A. V. Ambade, E. N. Savariar and S. Thayumanavan, Mol. Pharmacol., 2005, 2, 264 CrossRef CAS.
- R. M. Mainardes, M. C. C. Urban, P. O. Cinto, N. M. Khalil, M. V. Chaud, R. C. Evangelista and M. P. D. Gremiao, Curr. Drug Targets, 2005, 6, 363 Search PubMed.
- J. Alonso, Biomed. Pharmacother., 2004, 58, 168 Search PubMed.
- A. Enríquez de Salamanca, Y. Diebold, M. Calonge, C. García-Vazquez, S. Callejo, A. Vila and M. J. Alonso, Invest. Ophthalmol. Visual Sci., 2006, 47, 1416 CrossRef.
- M. M. Patel, J. D. Smart, T. G. Nevell, P. Eaton, R. J. Ewen and J. Tsibouklis, Biomacromolecules, 2003, 4(5), 1184 CrossRef CAS.
- E. R. Gillies and J. M. J. Frechet, Drug Discovery Today, 2005, 10, 35 CrossRef CAS.
- S. Svenson and D. A. Tomalia, Adv. Drug Delivery Rev., 2005, 57, 2106 CrossRef CAS.
- A. K. Patri, J. F. Kukowska-Latallo and J. R. Baker, Adv. Drug Delivery Rev., 2005, 57, 2203 CrossRef CAS.
- D. Cunliffe, A. Kirby and C. Alexander, Adv. Drug Delivery Rev., 2005, 57, 1836 CAS.
- P. J. Ginty, M. J. Whitaker, K. M. Shakesheff and S. M. Howdle, Mater. Today, 2005, 8, 42 Search PubMed.
- B. Sellergren and C. J. Allender, Adv. Drug Delivery Rev., 2005, 57, 1733 CrossRef CAS.
- A. G. Mayes and M. J. Whitcombe, Adv. Drug Delivery Rev., 2005, 57, 1742 CrossRef CAS.
- P. Pathak, M. J. Meziani and Y.-P. Sun, Expert Opin. Drug Delivery, 2005, 2, 747 Search PubMed.
- A. T. D. Silva, M. C. Chung, L. F. Castro, R. V. C. Guido and E. I. Ferreira, Mini-Rev. Med. Chem., 2005, 5, 893 Search PubMed.
- C. J. Hawker and K. L. Wooley, Science, 2005, 309, 1200 CrossRef CAS.
- M. Attar, J. Shen, K.-H. J. Ling and D. Tang-Liu, Expert Opin. Drug Delivery, 2005, 2, 891 Search PubMed.
- S. Dey and A. K. Mitra, Expert Opin. Drug Delivery, 2005, 2, 201 Search PubMed.
- M. E. Ganapathy and V. Ganapathy, Curr. Drug Targets Immune Endocr. Metabol. Disord., 2005, 5, 357 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |
