An efficient organogelator for ionic liquids to prepare stable quasi-solid-state dye-sensitized solar cells
Nils
Mohmeyer
a,
Daibin
Kuang
b,
Peng
Wang†
b,
Hans-Werner
Schmidt
*a,
Shaik M.
Zakeeruddin
*b and
Michael
Grätzel
*b
aMacromolecular Chemistry I, Bayreuther Institut für Makromolekülforschung (BIMF) and Bayreuther Zentrum für Grenzflächen und Kolloide (BZKG), University Bayreuth, D-95447 Bayreuth, Germany. E-mail: hans-werner.schmidt@uni-bayreuth.de; Fax: +49 (0) 921 55 3206; Tel: +49 (0) 921 55 3200
bLaboratory for Photonics and Interfaces, Swiss Federal Institute of Technology, CH-1015 Lausanne, Switzerland. E-mail: michael.graetzel@epfl.ch; shaik.zakeer@epfl.ch; Fax: +41 (0) 21 693 3115 (M. G.); Fax: +41 (0) 21 693 4111 (S. M. Z.); Tel: +41 (0) 21 693 3112 (M. G.) Tel: +41 (0) 21 693 6124 (S. M. Z.)
First published on 31st May 2006
Abstract
A low molecular weight amphiphilic organogelator capable of forming intermolecular hydrogen bonds and well-organized supramolecular structures was found to efficiently gel low-viscosity binary mixtures of ionic liquids at low concentrations. With this gelator it is possible to prepare stable quasi-solid-state dye-sensitized solar cells (DSCs). At a gelator concentration of only 2 wt%, the sol–gel transition temperature (Tgel) based on the lowest viscosity ionic liquid mixture was at 108 °C, well above the service temperature. Due to the thermoreversible nature of the system, the cells can be conveniently filled with a low-viscosity liquid. Upon cooling and formation of the gel a mechanically stable quasi-solid-state electrolyte was obtained. We successfully employed this quasi-solid ionic liquid electrolyte in DSCs and obtained an efficiency of 6.3% at full sunlight irradiation and maintained its stability during the light soaking accelerating stress test at 60 °C over 1000 h.
Introduction
Dye-sensitized solar cells (DSCs) are most attractive candidates due to their low production cost in comparison to silicon-based solar cells. Since the discovery of DSCs in 1991, many groups across the world have been working towards the development and optimization of DSCs.1 The nanoporous film texture of TiO2 in these cells has a large surface area to anchor photo-sensitizers and thus ensures efficient light-harvest and electron injection. Electron-transfer takes place from the excited dye molecules to the TiO2 where the electrons are transported to, and collected at, an electrode. Subsequent hole-transfer from the oxidized dye by the interception of iodide present in the electrolyte reduces the dye and in consequence the oxidized redox couple is reduced back at the counter electrode. Research is focused on the development of new dyes, semiconductor materials and redox electrolytes to enhance device performance and stability.2 However, encapsulation of organic solvent-based electrolytes in large scale modules remains an issue in view of a practical application. Thus, p-type inorganic semiconductors,3 organic hole-transport materials,4 and solvent-free polymer electrolytes incorporating triiodide/iodide as a redox couple5 were introduced to substitute for the liquid electrolytes.As part of the development of a stable electrolyte system, ionic liquids are screened as solvents due to their non-volatility, large electrochemical window and non-toxicity.6–9 As for organic solvents, ionic liquid systems were gelled using polymers, inorganic silica nanoparticles and low molecular weight organic gelators to make quasi-solid electrolytes.10–17 The low molecular weight organic gelators have the advantage, due to the thermoreversibility, that above the sol–gel transition temperature (Tgel), the nanopores can be efficiently filled with a low-viscosity liquid and upon cooling a mechanically stable quasi-solid electrolyte is obtained. In order to optimize such a system the Tgel has to be above 100 °C with the lowest possible gelator concentration.
The photovoltaic performance of ionic liquid-based electrolytes is lagging behind organic solvent-based systems due to their high viscosity. The viscosity of ionic liquid electrolytes can be reduced considerably by mixing high-viscosity iodide ionic liquids with low-viscosity ionic liquids containing weakly basic anions. Bearing this in mind, this work is based on a low-viscosity binary ionic liquid mixture of 1-propyl-3-methylimidazolium iodide (PMII) and 1-ethyl-3-methylimidazolium thiocyanate (EMINCS). Here we demonstrate that it is possible to gel this binary ionic liquid mixture with a special low molecular weight amphiphilic organogelator at concentrations as low as 2 wt% – a factor of two lower than the best reported organogelator for ionic liquid systems16 – and Tgel values above 100 °C. The prepared quasi-solid-state ionic liquid gel dye-sensitized solar cells are stable under light soaking at 60 °C for 1000 h and have an efficiency of 6.3% under simulated AM 1.5 full sunlight. The gelator has no influence on the performance of the solar cell. This is so far the best reported efficiency for a quasi-solid-state dye-sensitized solar cell based on ionic liquids with no organic solvent present.
Results and discussion
The gelation ability of low molecular weight organogelators in organic solvents depends strongly on the intermolecular forces of the gelator molecules and on the polarity of the applied solvent.18 The higher the polarity of the organic solvent the more complicated is the gelation due to the increased interactions with the gelator molecules. The gelator molecules often stay in solution. In apolar solvents the gelator molecules are difficult to dissolve, and in most cases the gelator precipitates out upon cooling. The gelator discussed here, cyclohexanecarboxylic acid-[4-(3-tetradecylureido)phenyl]amide (Fig. 1), is capable of efficiently gelling polar solvents such as γ-butyrolactone or valeronitrile at concentrations below 1 wt%.19 To illustrate its gelation capability in highly polar ionic liquids, Fig. 1 displays two vials with a mixture of two ionic liquids, PMII and EMINCS (65 ∶ 35 v/v). The left vial shows the pure ionic liquid mixture without the gelator. The liquid, upon turning the vial upside down, rapidly flows to the bottom of the vial. The right vial contains 2 wt% of the organogelator. After complete dissolution of the organogelator at elevated temperatures, the gelator molecules build up nano-scale fibrils due to the formation of hydrogen bonds and form a three-dimensional network. This leads to gelation of the ionic liquid mixture and hinders flow of the liquid.![Ionic liquid electrolyte for dye-sensitized solar cells based on the binary system of the ionic liquids 1-propyl-3-methylimidazolium iodide (PMII) and 1-ethyl-3-methylimidazolium thiocyanate (EMINCS) (65 ∶ 35 v/v). The left vial shows the liquid electrolyte, which upon turning the vial upside down rapidly flows to the bottom. With the addition of 2 wt% of the organogelator, cyclohexanecarboxylic acid-[4-(3-tetradecylureido)phenyl]amide, the electrolyte forms a stable gel (right vial).](/image/article/2006/JM/b604021g/b604021g-f1.gif) | ||
| Fig. 1 Ionic liquid electrolyte for dye-sensitized solar cells based on the binary system of the ionic liquids 1-propyl-3-methylimidazolium iodide (PMII) and 1-ethyl-3-methylimidazolium thiocyanate (EMINCS) (65 ∶ 35 v/v). The left vial shows the liquid electrolyte, which upon turning the vial upside down rapidly flows to the bottom. With the addition of 2 wt% of the organogelator, cyclohexanecarboxylic acid-[4-(3-tetradecylureido)phenyl]amide, the electrolyte forms a stable gel (right vial). | ||
At this point it should be mentioned that the addition of 0.12 M guanidinium thiocyanate, 0.5 M N-methylbenzimidazole and 0.2 M I2, which are added to the ionic liquid mixture for application in DSCs, does not change the gelation behavior or the Tgel values. As the additives do not influence the gelation behavior, the following investigations are discussed for the binary mixture of ionic liquids without the three additivies.
The formation of the supramolecular structures by the organogelator can be investigated using polarized optical light microscopy. Fig. 2 shows photographs from polarized optical light microscopy between crossed polarizers at different temperatures. To study the gelation mechanism, the system was first heated up to 160 °C to ensure complete dissolution of the gelator in the ionic liquid mixture. Subsequently, it was cooled to room temperature at a rate of 5 °C min−1. The upper photograph was taken on cooling to 110 °C and exhibits fine fibrillar birefringent structures, indicating that the gelator forms three-dimensional fibrillar structures in the ionic liquid. On cooling to 90 °C, further growth of the three-dimensional fibrillar structures of the gelator molecules was observed (Fig. 2 bottom).
 | ||
| Fig. 2 Optical micrographs between crossed polarizers at 110 and 90 °C showing the growth of fibrillar structures of the gelator in the ionic liquid mixture (PMII–EMINCS 65 ∶ 35 v/v). The scale bars are 100 µm. | ||
The morphology of the gels based on the binary system PMII–EMINCS (65 ∶ 35 v/v) comprising 2 wt% of the gelator was investigated by scanning electron microscopy (SEM) to prove the existence of the three-dimensional network structure in the ionic liquid. Fig. 3 displays two SEM pictures at different magnifications showing the morphology of the xerogel after extraction of the ionic liquids with liquid CO2. The top picture shows an open cellular morphology of the gelator composed of sheet-like objects. The bottom picture at a higher magnification reveals assembled stacks and a lamellar substructure (white arrow). The average thickness of each individual lamellar is between 60 and 80 nm.
 | ||
| Fig. 3 SEM pictures at different magnifications of xerogels of the organogelator after extraction of the ionic liquid mixture (PMII–EMINCS 65 ∶ 35 v/v) with liquid CO2 (initial gelator concentration: 2 wt%). | ||
After the characterization of the gel morphology, the following chapter describes the determination of Tgel by differential scanning calorimetry measurements at different compositions. Fig. 4 (top) shows the differential scanning calorimetry thermogram of the PMII ionic liquid gel with 2 wt% of the organogelator (upper curve) in comparison to the neat ionic liquid (bottom curve). The thermogram of the neat PMII displays no endothermic signal in the heating curve, whereas the curve of the gel shows an endothermic signal with its maximum at 119 °C corresponding to the gel-sol transition temperature. This means that below 119 °C the gel state is present and above this point the network collapses and a liquid is obtained. This high Tgel ensures the gel state of the electrolyte at the solar cell operating temperature.
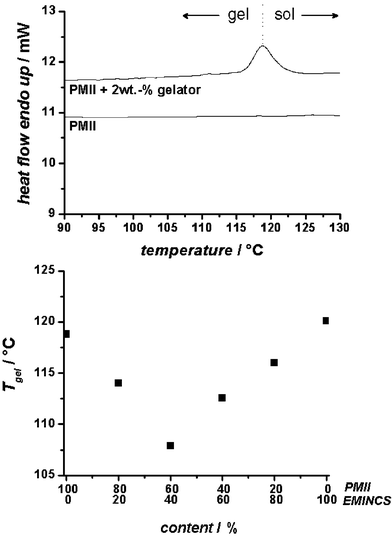 | ||
| Fig. 4 Top: Differential scanning calorimetric thermograms of PMII (bottom curve) and of the corresponding gel with 2 wt% of the gelator (top curve) showing the first order gel–sol transition. Bottom: Dependence of the Tgel values of gels with 2 wt% of the gelator as function of the composition of the ionic liquids (PMII and EMINCS). | ||
However, due to the strong ion pair effect of the iodide, the viscosity of PMII is relatively high, which results in lower efficiencies of the solar cell, because the charge diffusion is limited. An elegant way of reducing the viscosity of ionic liquid electrolytes is a binary mixture of PMII with a low-viscosity ionic liquid containing a weak basic anion. In the present work we have investigated mixtures with a low-viscosity ionic liquid, EMINCS, and PMII. Fig. 4 (bottom) shows Tgel as function of the ratio of the ionic liquids at an organogelator concentration of 2 wt%. As mentioned above, the Tgel of the neat PMII gel is at 119 °C. Interestingly, the Tgel of the low-viscosity neat EMINCS gel was found to be in the region of 120 °C. By adding EMINCS to PMII the Tgelvalues change significantly. Compared to the Tgel of the PMII gel at 119 °C, the Tgel values decrease with increasing amounts of EMINCS. From the investigated binary mixtures, the composition of 60 wt% PMII and 40 wt% of EMINCS has the lowest gel–sol transition temperature (108 °C). Further increases in EMINCS content lead to higher Tgel values of the binary ionic liquid mixtures.
A two-electrode-system electrochemical cell equipped with a Pt ultramicroelectrode (radius: 5 µm) as the working electrode and a Pt foil as the counter electrode was employed to measure the steady-state current for diffusion coefficient determination. The apparent diffusion coefficients (Dapp) of triiodide can be calculated from the steady-state current (Iss) according to eqn. (1),20
| Iss = 4ncaFDapp | (1) |
The overall power conversion efficiencies of devices with ionic liquid and gel electrolytes at different light intensities are listed in Table 1. Fig. 5 presents the photocurrent density-voltage curves for device A with gel electrolyte at irradiance of various light intensities and in the dark. The short-circuit photocurrent density (Jsc), open-circuit photovoltage (Voc) and fill factor (ff) of device A under AM 1.5 full sunlight are 12.7 mA cm−2, 706 mV, and 0.70, respectively, yielding an overall conversion efficiency (η) of 6.3%. The corresponding parameters (Jsc, Voc, ff, and η) of device B with a binary ionic liquid electrolyte without gelator are 12.8 mA cm−2, 687 mV, 0.72, and 6.3%, respectively. At various lower incident light intensities, the overall power conversion efficiencies even reach 7.5%.
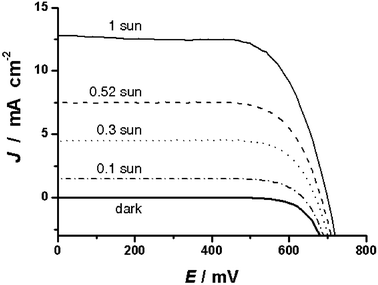 | ||
| Fig. 5 Photocurrent density-voltage characteristics of device A comprising 2 wt% of the gelator in the ionic liquid electrolyte at irradiance of various light intensities. Active area of devices with mask: 0.158 cm2. | ||
| Electrolyte | Device efficiency (%) at different incident light intensitiesa | |||
|---|---|---|---|---|
| 0.1 Sun | 0.3 Sun | 0.52 Sun | 1.0 Sun | |
| a The spectral distribution of the lamp mimics air mass 1.5 solar light. 1.0 Sun corresponds to an intensity of 100 mW cm−2. b 0.2 M I2, 0.12 M guanidinium thiocyanate, and 0.5 M N-methylbenzimidazole in the mixture of PMII and EMINCS (65 ∶ 35 v/v). c Same composition as (b) +2 wt% gelator. | ||||
| Ionic liquidb | 7.1 | 7.1 | 6.9 | 6.3 |
| Gelled ionic liquidc | 7.5 | 7.5 | 7.1 | 6.3 |
The cells were covered with a 50 µm-thick layer of polyester film (Preservation Equipment Ltd, UK) as a UV cut-off filter (up to 400 nm), and irradiated at open circuit and 60 °C under a Suntest CPS plus lamp (ATLAS GmbH, 100 mW cm−2). As shown in Fig. 6, device A showed an excellent stability under the dual stress of heating and visible light soaking, retaining 95.2% of its initial power conversion efficiency. Impressively, the measured Jsc of 13.1 mA cm−2 after 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h aging was still higher than the initial value of 12.7 mA cm−2, while a 35 mV drop in Voc and a decrease of less than 3% decrease in ff were observed.
000 h aging was still higher than the initial value of 12.7 mA cm−2, while a 35 mV drop in Voc and a decrease of less than 3% decrease in ff were observed.
 | ||
| Fig. 6 Top: Evolution of the device parameters Jsc, Voc and ff using quasi-solid ionic liquid gel electrolyte under a Sun light soaking at 60 °C. Bottom: Resulting overall solar conversion efficiency (η) as a function of time. | ||
Conclusions
In conclusion, we have demonstrated that an asymmetrical low molecular weight organogelator is capable of efficiently gelling a low-viscosity binary ionic liquid electrolyte. Tgel values well above 100 °C can be realized at a gelator concentration of only 2 wt%. The quasi-solid ionic liquid electrolyte gel was successfully employed in DSCs. The gelator does not interfere with and maintains the solar cell performance at the same level. We are confident that this type of gelator can also gel further optimized ionic liquid mixtures, which will be developed in the future at even lower concentrations.Experimental
Materials and methods
N-Methylbenzimidazole (NMBI) was purchased from Aldrich and recrystallized from diethyl ether before use. Ionic liquids PMII and EMINCS for the photovoltaic experiments were prepared according to the literature method8 and their purity was confirmed by 1H NMR spectroscopy. The ionic liquids (PMII and EMINCS) for the gelation experiments were obtained from IoLiTec, Freiburg, Germany and used as received. 400 nm-sized TiO2 particles were received as a gift from CCIC (Japan). The organogelator and K-19 dye were synthesized as reported earlier.19Voltammetric measurements
Voltammetric data were recorded at room temperature on an Autolab P20 electrochemical workstation (Eco Chimie, Netherlands). A two-electrode electrochemical cell, consisting of a 5.0 µm radius Pt ultramicroelectrode as the working electrode and a Pt foil as the counter electrode, was used for measurements of the triiodide diffusion coefficient in a binary ionic liquid electrolyte with and without gelator.Differential scanning calorimetry
T gel values of different mixtures of ionic liquids with and without gelator were determined using a differential scanning calorimeter (Diamond DSC, Perkin Elmer). The measurements were performed with samples of 20–40 mg at a heating rate of 10 °C min−1 and a cooling rate of 40 °C min−1 under nitrogen in a temperature range of 30 to 180 °C; annealing at 30 °C for 10 mins was carried out to ensure complete gelation of the mixture, followed by heating to 180 °C. The maximum of the second heating curve of the melting endotherm is listed as the Tgel value.Electron microscopy
For the scanning electron microscopy, a xerogel was accomplished. A 0.5 ml portion of gel from a mixture of the gelator (2.0 wt%) in PMII–EMINCS (65 ∶ 35 v/v) was prepared in a common snap cap glass vial with a diameter of 22 mm. After 48 h at room temperature, the glass vial was placed in an autoclave (volume: 1 liter; PARR Instrument Co., Frankfurt am Main). The autoclave was annealed at 8 °C with a pressure of 56 bar. After an extraction time of eight hours, the CO2 content was reduced to 10%. This process was repeated three times to ensure the complete extraction of the ionic liquid mixture. The autoclave was refilled with CO2, and after two hours the temperature was increased stepwise (2 °C every 10 min) up to 35 °C to reach a pressure of 130 bar in order to guarantee the parameters of the supercritical CO2. After 10 min, the pressure was reduced to 1 bar over one hour. The scanning electron microscopy measurements were performed with a LEO 1530 (FE-REM with Schottky-field-emision cathode) and an in-lens detector.Polarized optical light microscopy
For optical microscopic investigations, a piece of the gelled mixture (2 wt% of organogelator in the composition of the ionic liquids) was placed onto a glass slide and protected with a cover slip. The sample was heated to 160 °C and the micrographs were taken upon cooling between crossed polarizers using a Nikon microscope equipped with a hot-stage (Mettler Toledo FP82HT) at a standard cooling rate of 5 °C min−1.Preparation of double-layer TiO2 electrode
Fluorine-doped SnO2 conducting glass was first cleaned in Triton-100 aqueous solution, washed with ethanol, and treated with 50 mmol l−1 TiCl4 aqueous solution at 70 °C for 30 min to make a good mechanical contact between the printed TiO2 layer and the conducting glass matrix. Then, a 10 µm-thick film of 20 nm size TiO2 particles was printed on the treated conducting glass and further coated by a 4 µm-thick second layer of 400 nm light scattering anatase particles (CCIC, Japan). For the second layer, the screen-printing paste composed of 10 g of 400 nm sized TiO2 scattering particles and 2 g of 15 nm-sized TiO2 fine particles was used to obtain mechanically tough layer. The screen-printed layer was gradually heated up to 500 °C under oxygen and subsequently left for 10 min for sintering. The layer thickness was determined by Alpha-step 200 surface profilometer (Tencor Instruments, USA). After treating with 40 mmol l−1 TiCl4 again, the layer was rinsed with water and ethanol.Fabrication of dye-sensitized solar cells
After sintering at 500 °C and cooling down to 80 °C, the double-layer structured TiO2 electrode was dye-coated by immersing it in a 0.3 mM K-19 with 0.075 mM 1-decylphosphonic acid (DPA) in acetonitrile and tert-butanol (volume ratio: 1 ∶ 1) at room temperature for 12 h and then assembled with thermally platinized conducting glass electrodes. The devices A and B were fabricated based on the electrolytes containing 0.2 M I2, 0.12 M guanidinium thiocyanate, and 0.5 M N-methylbezimidazole in the mixture of PMII and EMINCS (65 ∶ 35 v/v) with (2 wt%) and without gelator, respectively. The electrodes were separated by a 35 µm-thick Bynel hot-melt ring (DuPont, USA) and sealed up by heating. The internal space was filled with electrolytes using a vacuum pump to produce devices A and B. The electrolyte-injecting hole made with a sand-ejecting drill on the counter electrode glass substrate was sealed with a Bynel sheet and a thin glass cover by heating.Photoelectrochemical measurements
A 450 W xenon light source (Oriel, USA) was used to give 100 mW cm−2 (the equivalent of one sun at AM 1.5) at the surface of the solar cells. The spectral output of the lamp was matched in the region of 350–750 nm with the aid of a Schott KG-5 sunlight filter (Präzisions Glas & Optik GmbH, Germany) so as to reduce the mismatch between the simulated and the true solar spectrum to less than 2%. Various incident light intensities were regulated with neutral wire mesh attenuators. The current–voltage characteristics of the cell under these conditions were obtained by external potential-bias to the cell and measuring the generated photocurrent with a Keithley model 2400 digital source meter (Keithley, USA). This process was fully automated using Wavemetrics software (http://www.wavemetrics.com/). A similar data acquisition system was used to control the incident photon-to-current conversion efficiency (IPCE) measurement. Light from a 300 W xenon lamp (ILC Technology, USA) was focused through a Gemini-180 double Monochromator (Jobin Yvon Ltd., UK) onto the photovoltaic cell under test. The monochromator was incremented through the visible spectrum to generate the IPCE (λ) as defined in eqn. (2) below,| IPCE (λ) = 1240(Jsc/λϕ) | (2) |
Acknowledgements
We thank Christian Wolf for assistance with the preparation of the xerogel, Clarissa Abetz (BIMF) for the scanning electron microscopy and P. Comte, R. Charvet for the TiO2 film fabrication. D. K., P. W., S. M. Z., M. G. thank the Swiss Science Foundation, and the Swiss Federal Office for Energy (OFEN), for the financial support.References
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS.
- M. Grätzel, Nature, 2001, 414, 338 CrossRef CAS; M. Grätzel, Chem. Lett., 2005, 34, 8 CrossRef.
- B. O'Regan, D. T. Schwartz, S. M. Zakeeruddin and M. Grätzel, Adv. Mater., 2000, 12, 1263 CrossRef CAS; G. R. A. Kumara, A. Konno, K. Shiratsuchi, J. Tsukahara and K. Tennakone, Chem. Mater., 2002, 14, 954 CrossRef CAS; B. O'Regan, F. Lenzmann, R. Muis and J. Wienke, Chem. Mater., 2002, 14, 5023 CrossRef CAS; T. Taguchi, X. Zhang, I. Sutanto, K. Tokuhiro, T. N. Rao, H. Watanabe, T. Nakamori, M. Uragami and A. Fujishima, Chem. Commun., 2003, 2480 RSC.
- U. Bach, D. Lupo, P. Comte, J. E. Moser, F. Weissörtel, J. Salbeck, H. Spreitzer and M. Grätzel, Nature, 1998, 395, 583 CrossRef; J. Krüger, R. Plass, M. Grätzel and H. J. Matthieu, Appl. Phys. Lett., 2002, 81, 367 CrossRef CAS; T. Kitamura, M. Maitani, M. Matsuda, Y. Wada and S. Yanagida, Chem. Lett., 2001, 1054 CrossRef CAS.
- A. F. Nogueira, J. R. Durrant and M. A. De Paoli, Adv. Mater., 2001, 13, 826 CrossRef CAS; T. Stergiopoulos, I. M. Arabatzis, G. Katsaros and P. Falaras, Nano Lett., 2002, 2, 1259 CrossRef CAS.
- N. Papageorgiou, Y. Athanassov, M. Armand, P. Bonhote, H. Petterson, A. Azam and M. Grätzel, J. Electrochem. Soc., 1996, 143, 3099 CAS.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley, Weinheim, 2002 Search PubMed.
- J. Dupont, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667 CrossRef CAS; W. Xu and C. A. Angell, Science, 2003, 302, 422 CrossRef CAS.
- P. Wang, S. M. Zakeeruddin, J.-E. Moser and M. Graetzel, J. Phys. Chem. B, 2003, 107, 13
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 CrossRef CAS.
280 CrossRef CAS. - W. Kubo, Y. Makimoto, T. Kitamura, Y. Wada and S. Yanagida, Chem. Lett., 2002, 948 CrossRef CAS.
- P. Wang, S. M. Zakeeruddin, I. Exnar and M. Graetzel, Chem. Commun., 2002, 2972 RSC.
- S. Murai, S. Mikoshiba, H. Sumino, T. Kato and S. Hayase, Chem. Commun., 2003, 1534 RSC.
- Y. Shibata, T. Kato, T. Kado, R. Shiratuchi, W. Takashima, K. Kaneto and S. Hayase, Chem. Commun., 2003, 2730 RSC.
- P. Wang, S. M. Zakeeruddin, P. Comte, I. Exnar and M. Graetzel, J. Am. Chem. Soc., 2003, 125, 1166 CrossRef CAS.
- M. A. Klingshirn, S. K. Spear, R. Subramanian, J. D. Holbrey, J. G. Huddleston and R. D. Rogers, Chem. Mater., 2004, 16, 3091 CrossRef CAS.
- W. Kubo, S. Kambe, S. Nakade, T. Kitamura, K. Hanabusa, Y. Wada and S. J. Yanagida, J. Phys. Chem. B, 2003, 107, 4374 CrossRef CAS.
- W. Kubo, T. Kitamura, K. Hanabusa, Y. Wada and S. Yanagida, Chem. Commun., 2002, 374 RSC.
- P. Terech and R. G. Weiss, Chem. Rev., 1997, 97, 3133 CrossRef.
- N. Mohmeyer and H.-W. Schmidt, Chem.–Eur. J., 2005, 11, 863 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, 2nd edn., Wiley, Weinheim, 2001 Search PubMed.
Footnote |
| † Present address: Cavendish Laboratory, Madingley Road, Cambridge, CB3 0HE, UK. |
| This journal is © The Royal Society of Chemistry 2006 |
