A symmetrical solid oxide fuel cell demonstrating redox stable perovskite electrodes
David M.
Bastidas
,
Shanwen
Tao
and
John T. S.
Irvine
*
School of Chemistry, University of St Andrews, Fife, Scotland, UK KY16 9ST. E-mail: jtsi@st-and.ac.uk
First published on 9th March 2006
Abstract
The perovskite (La0.75Sr0.25)Cr0.5Mn0.5O3 (LSCM) is shown to be an effective, redox-stable electrode that can be used for both cathode and anode SOFC operation, to provide a symmetrical fuel cell system with good performance characteristics.
Solid oxide fuel cells (SOFCs) are electrochemical devices which directly convert chemical energy to electricity with very high efficiency. Present development of SOFCs is mainly based on the yttria-stabilised zirconia (YSZ) electrolyte because it exhibits good thermal and chemical stability, high oxide-ion conductivity and mechanical strength at high temperature.1 The most commonly used anode materials for zirconia-based SOFCs are Ni/YSZ cermets, which display excellent catalytic properties for fuel oxidation and good current collection but do exhibit disadvantages, such as low tolerance to sulfur2 and carbon deposition3 when using hydrocarbon fuels and poor redox cycling causing volume instability. In previous studies we have sought to stabilise lanthanum manganite in fuel conditions by substitution with chromium. It has been found that the perovskite oxide (La0.75Sr0.25)Cr0.5Mn0.5O3−δ (LSCM) is an efficient oxide anode for SOFCs, which exhibits comparable anode performance to the traditional Ni-YSZ cermet.4–7
In a standard fuel cell, the anode and cathode are separated by a gastight electrolyte. The cathode in theory should only ever be exposed to an oxidising atmosphere; however, under load the electrode experiences more reducing conditions. The commonly used cathodes such as La0.8Sr0.2MnO3−δ (LSM)1 and La0.4Sr0.6Co0.8Fe0.2O3−δ (LSCF)8 or the recently reported Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF)9 are not stable in a strongly reducing atmosphere. When SOFCs are operated at lower cell voltage, pO2 at the triple phase boundary (TPB) of the cathode is quite low, which may cause redox degradation of the cathode and thus affect the long term stability of the cathode performance. This degradation would be minimised if a redox stable cathode could be utilised. A redox stable cathode is also a better choice for the traditional SOFCs in case some leakage might occur and fuel is introduced into the cathode side. Cathode materials for single chamber10,11 and flame12 SOFCs must be redox stable as well, because they are exposed to reducing atmospheres in operation.
In fabrication of ceramic devices such as SOFCs, the differences in thermal expansion coefficient and especially sintering activity between different components in the structure give rise to significant strains during manufacture. An SOFC with symmetrical electrodes will greatly reduce such strains and indeed enhance the ease of co-sintering. This would significantly reduce cost, and so is an important practical concept.
As (La0.75Sr0.25)Cr0.5Mn0.5O3−δ is a stable phase under oxidising conditions it seems possible that it may also be an effective cathode for SOFC operation. Previous work has investigated the effect of Cr substitution on the properties of (La,Sr)MnO3−δ finding that this significantly decreases electrical conductivity.13 This penalty may however be worth paying due to enhanced redox stability on Cr substitution. Furthermore this study13 demonstrated that a very small level of Cr substitution in the lanthanum manganite perovskite was very detrimental to conductivity and that this might be a factor contributing to the severe sensitivity of lanthanum manganite cathodes to Cr poisoning. Samples with higher Cr contents are less sensitive to Cr addition and hence might be more resistant to this Cr-poisoning effect.
Here we demonstrate that redox cycling of LSCM does not harm anode performance and further that LSCM is an effective cathode that is also redox stable. This makes it possible to manufacture symmetrical SOFCs using LSCM as both anode and cathode and indeed we show that this cell can be utilised in reversible mode.
Experimental
(La0.75Sr0.25)Cr0.5Mn0.5O3−δ was prepared by a combustion synthesis technique, employing the corresponding metal nitrates and ethylene glycol as precursors. La2O3 (Alfa, 99.99%) was dried, weighed and dissolved in dilute nitric acid to form lanthanum nitrate, then Sr(NO3)2 (Aldrich, 99+%), Cr(NO3)3·9H2O (Fisher, Acros, 99%) and Mn(Ac)2·4H2O (Acros, 99+%) were added to form a mixed nitrate solution of the required stoichiometry. Ethylene glycol was added at the rate of 800 ml for mole of product and the solution refluxed at 80 °C for two hours. After further concentration of the mixture at higher temperatures, a gel formed, combustion of which yielded a brown sponge-like material. Firing this above 1100 °C resulted in the formation of the sought perovskite.Electrochemical measurements were performed in two configurations, two electrode, whole cell tests and three electrode, half-cell polarisation tests. In all cases gas flows were approximately 100 ml min−1 and hence fuel/oxidant utilisation was less than 5%. For two electrode cell fabrication, LSCM powders pre-fired at 1200 °C for 10 hours were used to prepare an isopropanol based slurry for the electrode. The YSZ electrolyte was 20 mm in diameter and 0.2 mm in thickness. A multi-layer electrode was applied to decrease the series and polarisation resistances.5 The electrode with area of 1 cm2 and overall thickness of about 50 µm was deposited onto the YSZ electrolyte using an isopropanol-based slurry and firing typically at 1000–1300 °C. Graded electrodes were applied to optimise performance, the first layer was (La0.75Sr0.25)Cr0.5Mn0.5O3 70 wt%, YSZ 30 wt%, the second and the third layers were pure (La0.75Sr0.25)0.9Cr0.5Mn0.5O3. The first layer was dried and fired at 1000–1300 °C, then the second and the third layers were coated, dried and fired again. Gold mesh together with gold paste was applied on top of the working electrode as current collector. For three electrode tests,5 a 2 mm thick YSZ electrolyte was utilised with parallel working and counter LSCM electrodes as described above. For both working and counter, the electrode was not applied to the centre of the discs with a small circle uncovered in the centre. A Pt point reference was applied here on the counter electrode side.5
The impedance spectra of the electrochemical half-cell were recorded at open circuit voltage (OCV) with a 10 mV ac signal amplitude over the frequency range 105 to 0.01 Hz, using a Zahner IM6e. The cell performance was measured for the 2 electrode system using a Solartron 1287. Humidified 5% H2, H2 and reformate were fed to the cell as the fuel. The steam content was about 3% H2O, achieved by bubbling the gases through room temperature water. Humidified oxygen was passing over the cathode during the fuel cell tests.
Results
The structure of as synthesised (La0.75Sr0.25)Cr0.5Mn0.5O3−δ may be refined as rhombohedral with a = 5.4562(3) Å, α = 60.440(9)°, V = 116.00(4) Å, Z = 2. The phase seems stable under fuel conditions with a perovskite structure being retained on firing in 5% H2/3% H2O/92% Ar at 900 °C for 120 h, with the cell changing to an orthorhombic structure with a = 5.5028(4) Å, b = 5.4823(5) Å, c = 7.7686(1) Å, but with an increase in unit cell volume of up to 1%. This reduction correlates with about 0.25 oxygens per formula unit being lost from the oxidized form (δ ∼ 0) to the reduced form (δ ∼ 0.25).5 Structurally this perovskite therefore seems redox stable.A series of experiments were performed to investigate the influence of redox cycling upon electrochemical performance, looking for changes in the polarisation resistance at 900 °C, measured using 3-electrode half-cell techniques on redox cycling. Experiments were performed sequentially in humidified hydrogen, then in reformate and then switching to humidified oxygen, stabilising for 30–60 minutes in each atmosphere. The cycle was repeated 4 more times. The results for hydrogen atmosphere are presented in Fig. 1.
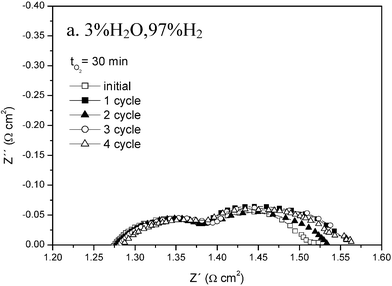 | ||
| Fig. 1 Half cell polarisation impedance plots in hydrogen atmospheres measured at 900 °C at zero bias showing changes over 5 redox cycles. | ||
The high frequency, low Z′ intercept relates to the series resistance primarily of the electrolyte and the low frequency, high Z′ intercept to the total of series and polarisation resistances. Two polarisation elements are present that we tentatively ascribe to electrochemical processes involving ion transport through the microstructure (high frequency) and charge transfer at the interfaces (low). The capacitances for these elements are of the order 7 × 10−4 F cm−1 and 5.51 × 10−3 F cm−1, consistent with this interpretation, and seem to rule out gas diffusion as being responsible.14 The high frequency element shows no significant change on cycling, whereas the low frequency element shows some slight initial degradation probably due to microstructural changes, but then stabilises. In the reformate the behaviour is similar although the diffusion element is larger. In unhumidified oxygen, Fig. 2, the two electrode arcs are merged. Again there is little evidence for degradation on cycling.
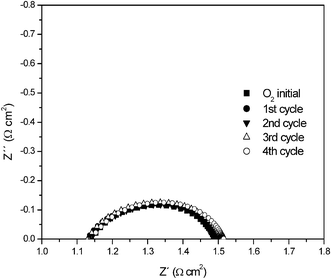 | ||
| Fig. 2 Half cell polarisation impedance plots in unhumidified oxygen atmosphere measured at 900 °C on cycling. | ||
The stability of these electrodes is quite remarkable. All experiments were performed at 900 °C with reduction and oxidation also occurring at this temperature, yet only minimal changes in electrode performance were detected.
Following on from these promising half-cell tests a symmetrical fuel cell was fabricated applying LSCM electrodes to both anode and cathode sides of a 200 µm thick yttria stabilised zirconia electrolyte. The cell performance at 900 °C is shown in Fig. 3 for different fuels at the anode with oxygen at the cathode. Wet H2 shows the highest performance due to its minimal dilution and also H2 is relatively easy to oxidise. A maximum power density of 300 mW cm−2 was achieved at 900 °C. A performance level between that of wet 5% H2 and wet H2 was achieved with wet methane as fuel and a maximum power density of 230 mW cm−2 was achieved. These are respectable performance levels at a self-supported electrolyte, higher values could be obtained at an electrode supported thin electrolyte.
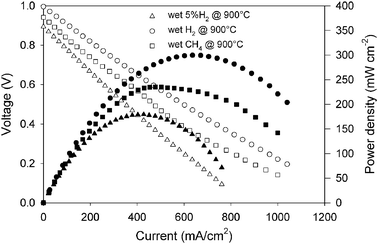 | ||
| Fig. 3 Fuel cell performance of symmetrical LSCM/YSZ/LSCM fuel cell for wet 5% H2, wet H2 and wet CH4. | ||
Since LSCM is an efficient redox stable electrode for both anode and cathode operation, it is possible to run the symmetrical cell as a reversible SOFC. Fig. 4 shows the performance of the symmetrical cell using wet H2 and wet O2 as fuel and oxidant respectively. The cell was successfully operated in both fuel cell and electrolyser modes. The area specific resistance in fuel cell mode is about 0.78 Ω cm2 and in electrolyser mode is 0.84 Ω cm2. The overall resistance of the cell working as electrolyser is just slightly higher (∼0.06 Ω cm2) than that running in fuel cell mode indicating that it is an efficient reversible SOFC. These area specific resistances measured under load match well with the constituent polarisation resistances displayed in Fig. 1, 2. The polarisation resistance in wet H2 is 0.3 Ω cm2, that in wet oxygen is 0.35 Ω cm2 and the series resistance for a 10 times thinner electrolyte than used in Fig. 1, 2 is about 0.13 Ω cm2. A small contribution to the series resistance may also come from the electrode, but this seems minor. A reversible fuel cell is particularly useful in combination with wind and wave energy technologies for the storage and utilisation of intermittent electricity supply.
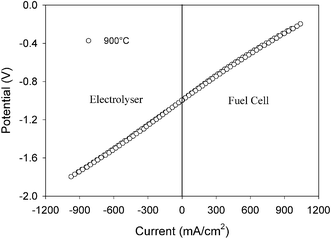 | ||
| Fig. 4 Current–voltage curve of the symmetrical cell LSCM/YSZ/LSCM under fuel cell and electrolyser modes in wet (2.5% H2O) H2versus wet O2. | ||
In conclusion, we have directly demonstrated that LSCM is both an efficient redox stable anode and an efficient redox stable cathode. With this particular property, it was possible to fabricate symmetrical SOFCs using LSCM for both electrodes and good performance was achieved. Since LSCM is stable and conductive in both reducing and oxidising atmospheres, the symmetrical cell may be operated in both fuel cell and electrolyser modes, i.e., as a reversible SOFC.
Acknowledgements
We would like to thank BOC Foundation, EPSRC and the EU Real SOFC Integrated Project for support and J. C. Ruiz-Morales for useful discussions.Notes and references
- N. Q. Minh, J. Am. Ceram. Soc., 1993, 76, 563 CAS.
- Y. Matsuzaki and I. Yasuda, Solid State Ionics, 2000, 132, 261 CrossRef CAS.
- B. C. H. Steele, I. Kelly, H. Middleton and R. Rudkin, Solid State Ionics, 28–30 Search PubMed.
- S. W. Tao and J. T. S. Irvine, Nat. Mater., 2003, 2, 320 CrossRef CAS.
- S. W. Tao and J. T. S. Irvine, J. Electrochem. Soc., 2004, 151, A252 CrossRef CAS.
- S. W. Tao, J. T. S. Irvine and J. A. Kilner, Adv. Mater., 2005, 17, 1734 CrossRef CAS.
- S. Zha, P. Tsang, Z. Cheng and M. Liu, J. Solid State Chem., 2005, 178, 1844 CrossRef CAS.
- H. Y. Tu, Y. Takeda, N. Imanishi and O. Yamamoto, Solid State Ionics, 1999, 117, 277 CrossRef CAS.
- Z. P. Shao and S. M. Haile, Nature, 2004, 431, 170–173 CrossRef CAS.
- T. Hibino, A. Hashimoto, T. Inoue, J. I. Tokuno, S. I. Yoshida and M. Sano, Science, 2000, 288, 2031 CrossRef CAS.
- Z. P. Shao, S. M. Haile, J. Ahn, P. D. Ronney, Z. L. Zhan and S. A. Barnett, Nature, 2005, 435, 795 CrossRef CAS.
- M. Horiuchi, S. Suganuma and M. Watanabe, J. Electrochem. Soc., 2004, 151, A1402 CrossRef CAS.
- R. Raffaelle, H. U. Anderson, D. M. Sparlin and P. E. Parris, Phys. Rev. B, 1991, 43, 7991 CrossRef CAS.
- M. J. Jorgensen and M. Mogensen, J. Electrochem. Soc., 2001, 148, A433 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
