Novel porous materials for emerging applications
Abstract
This issue of Journal of Materials Chemistry highlights recent research progress in synthesis, processing and characterization of novel porous materials for emerging applications. (George) X. S. Zhao, the Guest Editor, briefs the issue and provides an overview of the field.
A pore, which can be widely defined as a limited space or spatial confinement, is one of the most ancient concepts. The understanding, design, and manipulation of pores have significantly advanced science and technology, and are playing increasingly important roles in new technologies. For example, architectures can be considered as huge pores, in which human beings have been living in for thousands of years. The various containers in our daily life can also be thought of as big pores. The channels and interior spaces of biological membranes and tissues that facilitate the transport of water, ions and proteins are another sort of pores. The presence of holes in porous silicon can effectively emit visible light, thus the material can be used as optoelectronic switches, displays, and lasers.
According to the International Union of Pure and Applied Chemistry (IUPAC), pores are classified into three categories, namely micropore, mesopore, and macropore with pore sizes less than 2 nm, between 2 and 50 nm, and larger than 50 nm, respectively. Porous structures that can be of inorganic, organic, and inorganic–organic composite materials are of scientific and technological importance because of the ability of the pore wall to interact with atoms, ions, molecules and supermolecules, together with the capacity of controllable pore space to load or capture liquid and gas molecules, and solid particles. The tailorable pore size and pore wall surface make porous materials highly attractive in frontier research. The past decade has seen a number of significant breakthroughs in the design and processing of novel porous materials, driven by the rapid growth of emerging applications, such as energy conversion and storage, environmentally friendly catalysis, sensors, tissue engineering, DNA sequencing, drug delivery, medical diagnosis, cell-makers, and photonics. The emergence of such new technological applications requires a higher level of control over the porous properties of porous structures.
One of the symposia of the International Conference on Materials for Advanced Technologies 2005 (ICMAT2005) and the International Union of Materials Research Societies' 9th International Conference on Advanced Materials (IUMRS-ICAM) held in Singapore offered an opportunity for presenting and communicating recent research progress in novel porous materials for emerging applications. The porous materials discussed at the Symposium included silicates, organosilicas, silicon, alumina, aluminosilicates, metals, metal oxides, clay minerals, carbons and carbon nanotubes, polymers, and metal–organic frameworks of various forms such as nanoparticles, thin films, membranes, and monoliths. The feature and application articles published in this thematic issue of Journal of Materials Chemistry address and highlight the key recent advancements in this field. These articles specifically summarize the topics of templating-synthesis of porous materials, chemical modification of porous materials via molecular chemistry, construction of interior space of inorganic nanostructures via nano-building blocks, imprinting well-controlled closed-nanopores in spin-on polymeric dielectric thin films for the production of advanced integrated circuits (ICs), magnetophotonic crystals with artificial periodic structures for linear and nonlinear magneto-optical responses, synthetic routes to hierarchically structured meso/macroporous materials, and industrial applications of metal–organic frameworks. Further directions and key challenges in fundamental and applied research in the areas are highlighted.
Indeed, templating-synthesis strategies are becoming more and more popular for the preparation of novel porous materials, ranging from microporous to macroporous materials, and further to hierarchically structured porous materials. The templates that can be used include three types, namely soft, hard, and complex templates. The soft templates, which can be subsequently removed by heat treatment, are normally organic-based molecules, supramolecules, and molecular associations, such as organic amines, polymers, and surfactant micelles. Such soft templates have been employed in synthesizing microporous zeolites and ordered mesoporous molecular sieves. Of particular noteworthiness is the use of surfactant micelles to synthesize ordered mesoporous materials. The availability of the mesoporous materials has opened up unprecedented opportunities for dealing with large molecules such as biomolecules and heavy oils. Additionally, vesicles, ionic liquids, self-assembled colloidal crystals, and air bubbles that can be considered as soft templates have also been used to prepare various porous materials, including three-dimensional (3D) macroporous materials (the so-called inverse opals). The hard template, which can be leached away by using acid or alkali solution, actually refers to porous solids. If the pores are filled with a secondary material (sometimes coating technique is used to prepare more complex porous structures such as tubes), followed by a proper post-treatment and subsequent removal of the solid framework, an inverse structure of the same structural type to the solid is obtained. The hard template method has recently been employed to fabricate various novel porous structures, which are difficult to synthesize using the soft template method. For example, microporous zeolites, ordered mesoporous silicas (e.g., MCM-48, SBA-15, and SBA-16), and inverse opals have been used to fabricate microporous, mesoporous and macroporous carbons, respectively. The preparation of various porous metals and metal oxides with unique catalytic, sensoring, and electronic properties has been demonstrated using the hard template method. Here, it should be noted that anodic alumina membranes with micro- and sub-micrometre pores are another frequently used hard template for fabricating a wide spectrum of nanomaterials, such as quantum dots, wires, rods, and tubes. The complex template, which combines the soft template with the hard template, or two hard templates, or two soft templates of different length scales, has been used to prepare hierarchically bi-modal and tri-modal porous structures with the primary objectives of minimizing diffusion resistance and improving the accessibility of various surfaces. For examples, bimodal mesoporous–macroporous materials with interconnected pore channels can be prepared by using surfactant template in combination with a colloidal-crystal template. The surfactant template can also be combined with polymer foam, bio-cellulose, emulsion, inorganic salt, and ice crystal templates. Various meso–macroporous materials such as carbon, zirconia, titania, titanium phosphates as displayed in Fig. 1, as well as silica monolith with interconnected channels have been prepared by using this strategy.
 | ||
| Fig. 1 From Yuan and Su, Fig. 13, ref. 1 | ||
There has been growing research interest in the preparation of nanostructures of inorganic metals or metal oxides with controlled interior nanospace. Synthetic architectures with interior space has been built up using nano-units on the basis of various synthetic chemistry methodologies, including Ostwald ripening to build hollow anatase TiO2 spheres, hollow Cu2O spheres and core-shell ZnS spheres, Kirkendall diffusion to construct ZnO hollow dandelions, the oriented attachment principle to make CuO hollow dandelions and hollow octahedral SnO2, the space-predefined growth route to fabricate crystalline cuprous oxides with intra-crystal porosity, and a combined strategy to fabricate Au–TiO2 complex nanocomposites with an Au core and an anatase TiO2 shell as shown in Fig. 2. Such novel synthetic strategies have opened new avenues to preparation of architectures of discrete but complex nanocomposites with interior space and high process flexibility, which can be potentially applied in optical, electronic, magnetic, catalytic and sensing devices ranging from photonic crystals to drug-delivery carriers and nanoreactors.
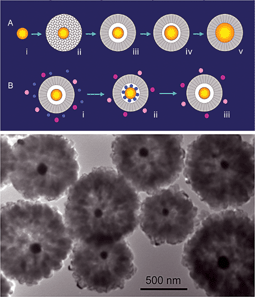 | ||
| Fig. 2 From Zeng, Fig. 9, ref. 2 | ||
A new family of nanoporous materials, called metal–organic framework (MOF) has significantly advanced the understanding of rational design and synthesis of porous materials (see Fig. 3). MOF materials possess unique physical and chemical characteristics that can not be observed from other inorganic and organic porous materials. The metal ions, acting as the coordination centres, can link with a variety of polyatomic organic bridging ligands, leading to the formation of MOF materials. With the concept of “reticular design”, various MOF materials with pore sizes ranging from the micropore to the mesopore scales with a high surface area have been described. Importantly, the metal sites are fully exposed and usually behave differently from bulk metals, thus creating a high degree of metal dispersion and various functionalities for catalysis and adsorption. Many different types of MOF materials are available now. Industrial applications of MOFs have been exemplified by the scientists of BASF in catalysis (formation of methoxypropene from propyne, vinylester synthesis from acetylene), gas purification (traces of tetrahydrothiophene in methane), pressure swing separation of rare gases (krypton and xenon), and physical storage of hydrogen.
 | ||
| Fig. 3 From Mueller et al., Fig. 2, ref. 3 | ||
The well-controlled nanopores imprinted in spin-on polymeric dielectric thin films play an important role in lowering the dielectric constant (low-k) of electric materials. Closed nanopores are prone to prevent metals (e.g., copper) from diffusion into the interdielectric layers during the fabrication process of integrated circuits (ICs). The dispersion to the dielectric precursor with hollow closed silica nanoparticles and nanoscale porogens (such as dendrimers, star-shape polymers, hyperbranched polymers, cross-linked polymer nanoparticles, core–corona polymer nanoparticles, linear polymers, cage supramolecules, hybrid organic–inorganic copolymers, and hybrid organic–organic copolymers) is effective to imprint closed-nanopores. However, significant challenges remain in the fabrication and production of high-performance low-k dielectric materials consisting of closed nanopores of 5 nm or less that meet the requirements of the production of advanced ICs in the microelectronics industry.
I hope that the excellent feature and application articles presented in this themed issue will offer a valuable reference for the porous materials society. I also encourage readers to consult with other references devoted to porous materials. I would like to thank the authors of the feature and application articles for their contributions. I would also like to thank Mr Fabing Su, National University of Singapore, Rebecca Gillan and Graham McCann of the RSC for their kind assistance and dedication to this themed issue.
 | ||
| Plate1 | ||
(George) X. S. Zhao,
National University of Singapore.
References
- Z.-Y. Yuan and B.-L. Su, J. Mater. Chem., 2006, 16 10.1039/b512304f.
- H. C. Zeng, J. Mater. Chem., 2006, 16 10.1039/b511296f.
- U. Mueller, J. Pastre, H. Puetter, M. Schubert, K. Schierle-Arndt and F. Teich, J. Mater. Chem., 2006, 16 10.1039/b511962f.
| This journal is © The Royal Society of Chemistry 2006 |
