Deposition of stable hydrophobic coatings with in-line CH4 atmospheric rf plasma
Jeong-Hoon
Kim
a,
Guangming
Liu
b and
Seong H.
Kim
*b
aCJ Creations, P.O. Box 82, Port Matilda, PA 16870, USA
bDepartment of Chemical Engineering, The Pennsylvania State University, University Park, PA 16802, USA. E-mail: shkim@engr.psu.edu
First published on 11th January 2006
Abstract
A CH4 atmospheric rf plasma treatment process was demonstrated for hydrophobic treatments of various substrates including metallic and insulating surfaces as well as flat and rough surfaces. A stable rf glow plasma was generated over a 1 cm × 16 cm area using a cylindrical electrode geometry. A typical hydrophobic treatment speed was 5–10 cm min−1. CH4 plasma polymerization deposited a very smooth hydrocarbon layer composed of CH2 and CH3 groups. The inclusion of oxygenated species was less than 1%. On flat substrates, the water contact angle was ∼90°. On rough surfaces such as cotton, the contact angle reached up to 150°. Since this method does not require vacuum or any special venting systems, it will be suitable for in-line processing of a wide range of substrate materials.
Introduction
Deposition of hydrophobic coatings on various materials has many important applications such as protective garments, water repellent textiles, corrosion prevention, micro-device lubrication, microfluidics, barrier coatings in biomedical systems, etc.1–7 Among many techniques that can be utilized for this purpose, a plasma-based process is very attractive in many aspects.8–12 In general, plasma-based treatment methods do not produce chemical wastes and waste disposal problems and they can be fully automated. They give a high efficiency of surface activation for various materials by actions of UV photons, radicals and charged particles (electrons and ions). In addition, the plasma treatments do not alter the mechanical properties of bulk materials.13Applications of plasma-based techniques to coating processes have recently made significant progress. In the past, a majority of the plasma-based processes developed were vacuum plasma;8–12 so they were not suitable for continuous in-line manufacturing. In the batch process, the sample transfer into and out of a vacuum system is quite inconvenient and slow. For this reason, various types of atmospheric plasma processes have been demonstrated;14–19 but the plasma stability and low power operation still remain as challenges that need to be solved for practical applications. Unlike vacuum plasma, the gas consumption rate is large in atmospheric plasma. So the process gases must be cheap and stable.
This paper demonstrates the CH4 plasma polymerization deposition of stable hydrocarbon coating layers using an in-line atmospheric rf plasma process. An atmospheric rf glow-discharge plasma was generated over 1 cm × 16 cm with a cylindrical electrode. Hydrophobic coating layers were produced by plasma polymerization of CH4. The substrate was continuously translated under the plasma deposition region at a speed of 5–10 cm min−1. The atmospheric CH4 plasma process was able to treat both conducting and insulating substrates under the same operation conditions. Even though the plasma deposition process was operated in atmospheric conditions, the incorporation of oxygenated species was negligible. Ultrahydrophobicity was attained on rough surfaces such as cotton.
Experimental
The atmospheric rf glow-discharge plasma system was constructed with a custom-made plasma generator head (Changjo Engineering, Korea) and a 13.56 MHz rf supply with a L-C matching unit (Seren Industrial Power Systems). A schematic of the plasma head and sample stage is shown in Fig. 1. The plasma discharge characteristics are similar to those reported in ref. 20. The atmospheric plasma was operated in a glow discharge mode; so it could be directly applied to metallic substrates as well as non-conducting substrates without arc or streamer damage. The usable plasma area is 1 cm wide and 16 cm long. Helium or argon was used as a carrier gas (5–10 l min−1) and CH4 was used as a reactive gas (10–40 sccm). The rf power was controlled in the range of 250–400 W. Samples were mounted on a computer-controlled moving stage that traveled about 0.3–0.5 cm below the plasma source along the orthogonal direction to the plasma source head. In typical processes, the substrate was repeatedly passed back and forth across the glow-discharge plasma region at a speed of 5–10 cm min−1. The substrates tested in this work were clean Si wafers, gold films, copper foils, glass slides, papers, and cottons (Oxford cloth, 20 thread counts per centimeter). An oxygen plasma cleaning process was applied to Si, gold, copper, and glass samples prior to hydrophobic treatments in order to remove any organic contaminants on the surface. Paper and cotton samples were used without any plasma cleaning process. | ||
| Fig. 1 Schematic view of atmospheric rf plasma source and sample mounting stage. | ||
The plasma-deposited coatings were analyzed with X-ray photoelectron spectroscopy (XPS), polarization modulation reflection–absorption infrared spectroscopy (PM-RAIRS), atomic force microscopy (AFM), and sessile drop contact angle measurements. XPS analysis was performed with a VG ESCA MK-II system. A Mg source was used. Surface charging was corrected by shifting the C 1s peak to 285.0 eV. PM-RAIRS spectra of hydrophobic coatings on a gold film substrate were collected with a Thermo-Nicolet Nexus 670 spectrometer with a HINDS polarization modulation system. IR spectra for coatings on other substrates could not be obtained since the coating thickness was too small. The deposited coating layer thickness was measured with a Gaertner Scientific L116C ellipsometer. For the thickness measurement, a clean Si wafer was used as a substrate. AFM images of the hydrophobic coatings deposited on flat substrates were obtained with a Molecular Imaging SPM unit equipped with a RHK Technology controller. Topographic images were obtained by a contact mode scanning. Water contact angles were taken with a home-made sessile drop system. The contact angle was determined by calculating the slope of the tangent to the drop at the liquid–solid–vapor interface line.
Results and discussion
The chemical composition of the deposited hydrophobic coatings is determined using XPS. Fig. 2 shows the XPS analysis results for the coating deposited on a clean gold film substrate. Although the chemical bonding of the first layer to the substrate might be different depending on the substrate, the multilayer chemistry will be independent of the substrate properties and its chemical composition will be identical as long as the plasma conditions are the same. As expected, hydrophobic coatings deposited on other substrates revealed essentially the same features in XPS. For all substrates tested in this study, the substrate peak is significantly suppressed even after a single treatment and decreased to a very low signal-to-noise level after treatment with plasma three times. In each plasma treatment cycle, the C1s peak was the only main peak detected in XPS. The high resolution C1s peak region (Fig. 2, inset) shows a single peak at 285 eV with a small asymmetric tail on the high energy side. The high energy tail is due to oxygenated species. The elemental analysis shows that the oxygen concentration is always less than 2% regardless of the substrate. The incorporation of this small amount of oxygenated species is inevitable because the plasma is generated in atmospheric air. | ||
| Fig. 2 Survey XPS spectrum and high resolution C1s and O1s XPS spectra of the hydrophobic coating layer deposited on a gold film substrate with CH4–He atmospheric plasma. The coating was made by passing the substrate under the plasma region three times at a speed of 6 cm min−1. | ||
The exact nature of the carbonaceous species was determined with infrared vibration spectroscopy. Fig. 3 displays PM-RAIRS spectra of the hydrophobic coatings deposited on a gold film as a function of plasma treatment cycles. After the clean gold substrate is exposed to the plasma deposition process once, there are well defined C–H vibration peaks appearing at 2958 cm−1, 2930 cm−1, 2871 cm−1, 2840 cm−1 (as a shoulder), 1460 cm−1, and 1378 cm−1, as well as broad peaks at 1708 cm−1 and 1660 cm−1. These spectra are quite similar to the IR spectrum of the hydrocarbon coating deposited via a vacuum CH4 plasma process.21 The intensities of all peaks increase in proportion to the number of plasma deposition cycles. The peaks at 1708 cm−1 and 1660 cm−1 are due to carbonyl containing groups which are inevitable because the plasma is generated in atmospheric air. The reason that the carbonyl peak looks larger in IR than XPS is because the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration has a much higher IR absorption cross-section than the C–H vibrations. Other C–H vibration peaks are compared favorably to the IR spectra of polypropylene (Table 1). Compared to the polypropylene IR spectrum, the CH2 asymmetric and symmetric stretch intensities are slightly smaller and the CH2 deformation intensity is a little bit larger. Apart from these minor differences in peak intensities, the peak positions match up very closely. These results indicate that the atmospheric CH4 plasma polymerization deposits a hydrophobic coating composed mostly of CH2 polymeric backbones with CH3 side groups.
O vibration has a much higher IR absorption cross-section than the C–H vibrations. Other C–H vibration peaks are compared favorably to the IR spectra of polypropylene (Table 1). Compared to the polypropylene IR spectrum, the CH2 asymmetric and symmetric stretch intensities are slightly smaller and the CH2 deformation intensity is a little bit larger. Apart from these minor differences in peak intensities, the peak positions match up very closely. These results indicate that the atmospheric CH4 plasma polymerization deposits a hydrophobic coating composed mostly of CH2 polymeric backbones with CH3 side groups.
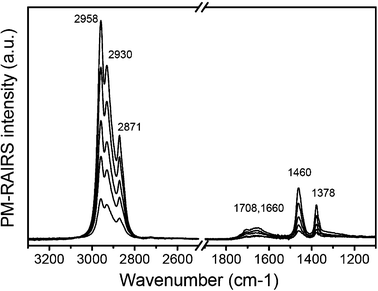 | ||
| Fig. 3 PM-RAIRS spectra of the hydrophobic coating layer deposited from the CH4–He plasma as a function of the number of passes. The plasma passes are 1, 3, 5, 7, and 9 from the lowest to highest intensity. The pass speed was 6 cm min−1. | ||
| Hydrophobic coating deposited via atmospheric CH4 plasma | Polypropylene | Vibration assignment |
|---|---|---|
| 2958 cm−1 | 2955 cm−1 | CH3 asymmetric stretch |
| 2930 cm−1 | 2925 cm−1 | CH2 asymmetric stretch |
| 2871 cm−1 | 2873 cm−1 | CH3 symmetric stretch |
| 2840 cm−1 | 2840 cm−1 | CH2 symmetric stretch |
| 1708 cm−1 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch O stretch |
|
| 1660 cm−1 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch O stretch |
|
| 1460 cm−1 | 1460 cm−1 | CH2 deformation |
| 1378 cm−1 | 1377 cm−1 | CH3 deformation |
The thickness of the hydrophobic coatings deposited with the atmospheric CH4 rf plasma was measured with ellipsometry. Fig. 4 plots the coating thickness as a function of plasma treatments for a processing rate of 6 cm min−1. The coating thickness is very uniform over the entire treated area. The thickness varies only 2–6% from the mean value, which could be just a measurement error. The deposition rate is ∼14 nm for the first pass and then slightly decreases in subsequent passes. It is speculated that as the coating layer becomes thicker, the degree of surface charging increases and the number of ions and/or electrons hitting the surface decreases. This would result in lowering the surface activation efficiency by the charged particle impingements, which would in turn cause a lower probability for reactions with radical species.
 | ||
| Fig. 4 Thickness of the deposited coating layer as a function of the CH4–He plasma treatments. Each treatment was made at a pass speed of 6 cm min−1. | ||
The atmospheric rf plasma deposition of polymeric hydrocarbon coatings gives quite smooth surface topography. Fig. 5 compares the topographic images and line profiles of a clean Si wafer with those treated with the atmospheric CH4–He plasma 3 and 11 times. The root-mean-square surface roughness (measured for 1.5 µm × 1.5 µm) slightly increases from 1.57 Å for the clean Si wafer to 4.71 Å for the sample deposited 3 times and 5.08 Å for the sample deposited 11 times. The roughness is not a strong function of the deposition cycle. The largest topographic features are a 3 nm tall and 150 nm wide lump in Fig. 5(b) and a 3 nm deep and 200 nm wide hole in Fig. 5(c). Other than these defective spots, the entire surface remains quite smooth, regardless of the rf deposition cycle.
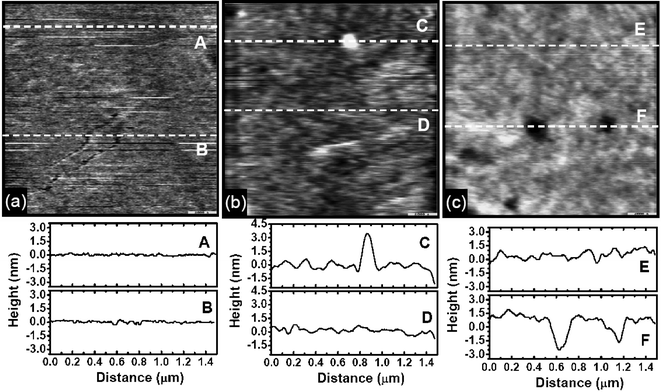 | ||
| Fig. 5 Contact mode AFM images of (a) a clean Si wafer and coatings deposited by (b) 3 times and (c) 11 times of the CH4–He plasma passing. The passing speed was 6 cm min−1. Line profiles of the marked locations are also shown. | ||
All coatings deposited by the atmospheric CH4–He plasma process show good hydrophobicity regardless of the substrate materials. Fig. 6 displays optical images of water droplets placed on these coatings produced on Si water, Cu foil, paper, and cotton substrates. Water contact angles on these coatings are plotted in Fig. 7 as a function of the plasma deposition cycle. On flat surfaces such as Cu foil, Si wafer, and glass slide, the contact angle increases to ∼90° after a single treatment with the CH4 plasma. This value is very close to the water contact angle of polypropylene (93°). The contact angle remains unchanged upon further deposition. The paper and cotton substrates require at least 3 deposition cycles to be hydrophobic. Until then, the water droplet is completely absorbed into the substrate. This probably arises from lower deposition yields on side walls present in rough surfaces, so repeated treatments are required for conformal coating. It should be noted that the contact angles on paper (112.5 ± 1°) and cotton (150 ± 1°) substrates are significantly higher than those on flat substrates. The higher contact angle on these substrates is due to the surface roughness effect that amplifies the hydrophobicity.22,23 In general, the water contact angle becomes larger on rough surfaces than on flat surfaces. On the treated cotton sample, the water droplet is easily rolled off with slight tilting of the substrate, showing a self-cleaning capability. It should be noted that the untreated side of the cotton sample still absorbs water readily. These properties will make the plasma-treated cotton fabric a good candidate for self-cleaning garments.
 | ||
| Fig. 6 Optical images of water droplets placed on hydrophobic coatings deposited on (a) Si wafer, (b) Cu foil, (c) paper, and (d) cotton substrates. | ||
 | ||
| Fig. 7 Water contact angle on hydrophobic coatings deposited on Si wafer (▼), copper (▲), glass (■), paper (●), and cotton (✦) substrates as a function of the number of plasma passes. | ||
The atmospheric rf glow-discharge plasma described in this paper is suitable for continuous in-line manufacturing. It can be applied to any surface regardless of the surface chemistry of the substrate. The deposited hydrophobic coating is very stable and durable. There is no noticeable degradation of the hydrophobicity for the samples exposed in air for long periods of time (we tested up to 4 months). The plasma-deposited coatings remain intact even in organic solvents such as hexane and retain the hydrophobicity after the solvent is dried completely. The coating on cotton fabric does not degrade even after many cycles of cold water washing (without mechanical rubbing).
Conclusions
A CH4 atmospheric rf plasma treatment process has been developed which can be applied to various substrates regardless of their surface roughness and chemistry. Since the plasma is operated in the glow discharge region, it can be applied directly to both metallic and insulating substrates. The atmospheric operation and use of stable hydrocarbon gas make it suitable for continuous in-line processing. The plasma polymerization of CH4 produces a very smooth and stable hydrocarbon coating composed of CH2 and CH3 groups. The water contact angle of the produced coating is ∼90° on flat surfaces and reaches up to 150° on rough surfaces such as cotton.Acknowledgements
The authors acknowledge Changjo Engineering and Seren Industrial Power Systems for lending their instruments for this experiment. We also acknowledge Professor Sae-Hoon Kim for helping us with instrument loan from Changjo Engineering. This work is supported in part by the 3 M Non-tenured Faculty Grant.References
- Q. D. Xie, G. Q. Fan, N. Zhao, X. L. Guo, J. Xu, J. Y. Dong, L. Y. Zhang, Y. J. Zhang and C. C. Jan, Adv. Mater., 2004, 16, 1830 CrossRef CAS.
- M. Thieme, R. Frenzel, S. Schmidt, F. Simon, A. Hennig, H. Worch, K. Lunkwitz and D. Scharnweber, Adv. Eng. Mater., 2001, 3, 691 CrossRef CAS.
- J. Y. Shiu, C. W. Kuo, P. L. Chen and C. Y. Mou, Chem. Mater., 2004, 16, 561 CrossRef CAS.
- M. B. Olde Riekerink, G. H. M. Engbers, M. Wessling and J. Fei, J. Colloid Interface Sci., 2002, 245, 338 CrossRef CAS.
- M. Ibnabddjalil, I.-H. Loh, C. C. Chu, N. Blumenthal, H. Alexander and D. Turner, J. Biomed. Mater. Res., 1994, 28, 289 CrossRef CAS.
- L. Zhai, F. C. Cebeci, R. E. Cohen and M. F. Rubner, Nano Lett., 2004, 4, 1349 CrossRef CAS.
- K. K. S. Lau, J. Bico, K. B. K. Teo, M. Chhowalla, G. A. J. Amaratunga, M. W. Milne, G. H. McKinley and K. K. Gleason, Nano Lett., 2003, 3, 1701 CrossRef CAS.
- I. Woodward, W. C. E. Schofield, V. Roucoules and J. P. S. Badyal, Langmuir, 2003, 19, 3432 CrossRef CAS.
- J. K. Evju, P. B. Howell, L. E. Locasicio, M. J. Tarlov and J. J. Hickman, Appl. Phys. Lett., 2004, 84, 1668 CrossRef CAS.
- S. M. Mukhopadhayay, P. Joshi, S. Datta and J. Macdaniel, Appl. Surf. Sci., 2002, 201, 219 CrossRef.
- C. L. Rickette, A. E. Wallis, J. C. Whitehead and K. Zhang, J. Phys. Chem. A, 2004, 108, 8341 CrossRef.
- W. Leahy, V. Barron, M. Buggy, T. Young, A. Mas, F. Schue, T. McCabe and M. Bridge, J. Adhesion, 2001, 77, 251 Search PubMed.
- Z. S. Cai, Y. P. Qiu, C. Y. Zhang, Y. J. Hwang and M. McCord, Textile Res. J., 2003, 73, 670 Search PubMed.
- L. J. Ward, W. C. E. Schofield and J. P. S. Badyal, Langmuir, 2003, 19, 2110 CrossRef CAS.
- M. G. McCord, Y. J. Hwang, Y. Qiu, L. K. Canup and M. A. Bourham, J. Appl. Polym. Sci., 2003, 88, 2038 CrossRef CAS.
- S. Guimond and M. R. Wertheimer, J. Appl. Polym. Sci., 2004, 94, 1291 CrossRef CAS.
- X. Yang, M. Moravej, S. E. Babayan, G. R. Nowling and R. F. Hicks, Plasma Sources Sci. Technol., 2005, 14, 412 CrossRef CAS.
- H. W. Herrmann, I. Henins, J. Park and G. S. Selwyn, Phys. Plasma, 1999, 6, 2284 Search PubMed.
- T. Yamamoto, M. Okubo, N. Imai and Y. Mori, Plasma Chem. Plasma Proc., 2004, 24, 1 Search PubMed.
- S. Y. Moon, W. Choi and B. K. Kang, Appl. Phys. Lett., 2004, 84, 188 CrossRef CAS.
- J. Hopkins and J. P. S. Badyal, Langmuir, 1996, 12, 4205 CrossRef CAS.
- R. N. Wenzel, Ind. Eng. Chem., 1936, 28, 988 CrossRef CAS.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546 RSC.
| This journal is © The Royal Society of Chemistry 2006 |
