Synthesis of copper/cross-linked poly(vinyl alcohol) (PVA) nanocables via a simple hydrothermal route
Junyan
Gong†
ab,
Linbao
Luo†
ab,
Shu-Hong
Yu
*ab,
Haisheng
Qian
ab and
Linfeng
Fei
b
aDivision of Nanomaterial & Chemistry, Hefei National Laboratory of Physical Sciences at microscale, School of Chemistry and Materials, University of Science and Technology of China, Hefei, 230026, P. R. China. E-mail: shyu@ustc.edu.cn; Fax: +86 551 3603040
bStructure Research Laboratory of CAS, University of Science and Technology of China, Hefei, 230026, P. R. China
First published on 14th October 2005
Abstract
Cross-linking reaction of poly(vinyl alcohol) (PVA) can be initiated in the presence of copper ions, resulting in the formation of copper@cross-linked PVA nanocables by a one-step hydrothermal approach. In contrast to our previous findings in the case of silver ions, metal ions with high valency are more difficult to reduce during the cross-linking reactions under hydrothermal conditions. The variation of pH value during the reaction has significant effects on the quality of the product. Copper@cross-linked PVA nanocables with a diameter of 0.5–1 µm and length up to 100 µm can be obtained at 200 °C, accompanying the presence of some cross-linked PVA aggregates with near spherical shape and irregular shape. The pH value, reaction temperature, and reaction time play key roles in the formation of such nanocables. The results demonstrated that the cross-linking reaction in the presence of different metal ions has different reaction rates, which determine the uniformity of the product and the quality of the cable-like core–shell structures.
Introduction
One-dimensional (1D) nanostructures have become the focus of considerable research in fabricating nanoscale electronic, optoelectronic and magnetic devices,1–4 which also provide an ideal model to experimentally investigate physical phenomena such as quantized conductance and size effects. As a kind of new nanostructure, core–shell nanostructures have received intense attention duo to their improved physical and chemical properties over their single components, and thus many efforts have been made to synthesize such special core—shell nanostructures.5Recently there have been a few reports on the preparation of semiconductor/insulator nanocables. For example, Si/SiO2 nanocables have been prepared by combining laser-ablation cluster formation with vapor–liquid–solid (VLS) growth.6 Silicon nanowires in graphitic B–C–N nanotubes can be prepared by a high temperature approach,7 and β-SiC/SiO2 nanocables have been obtained by the carbothermal reduction of sol–gel derived silica xerogels containing carbon nanoparticles at 1650 °C.8 Furthermore, CdSe/poly(vinyl acetate) hybrid nanocables have been obtained by growing the semiconductor nanowires inside polymer tubules.9
Metal/insulator nanocables represent another kind of new nanostructure, their syntheses also have attracted a lot of recent efforts. Ag/SiO2 nanocables can be formed using a sol–gel method to coat Ag nanowires with amorphous silica.10 Copper is malleable, ductile, and a good conductor of heat and electricity (second only to silver in electrical conductivity), which is most commonly used as an interconnector due to its high electrical conductivity and the availability of copper nanowires with well-defined dimensions should be able to bring in new types of applications or enhance the performance of currently existing electric devices.11 However, searching for new synthetic strategies for fabrication of nanocables has been a hot topic. Recently, we have proposed facile hydrothermal approaches for the synthesis of silver@cross-linked poly(vinyl alcohol)12 and silver@carbon nanocables13 using PVA and starch as shell precursors, respectively.
In this paper, we examine the versatility and capability of this approach and explore whether other high valent ions such as Cu2+ can be reduced to form Cu@cross-linked PVA cables. The results have demonstrated that Cu@cross-linked PVA nanocables can also be synthesized by controlling the reaction conditions, such as pH value, reaction temperature, and reaction time.
Experimental
Materials
All of the chemical regents used in this study are analytical grade and used without further purification. The reactants are CuCl2·2H2O (0.6 mmol), PVA solution (3 wt%, 6 mL) and NaOH solution (1.25 M, 3 ml).Synthesis procedures
The copper@cross-linked PVA nanocables were synthesized by reduction of Cu2+ in a PVA solution. In a typical procedure, CuCl2·2H2O (0.6 mmol) and 6 mL of PVA (3 wt%) solution respectively were dissolved in 10 ml water, then CuCl2 solution was added slowly dropwise to the PVA solution. The pH value of the reaction solution was adjusted to 11.3 with NaOH solution, followed by stirring for 30 min. Finally the whole solution was transferred into a Teflon-lined autoclave with a volume of 30 ml. After the autoclave was sealed, it was heated and maintained in an oven at 200 °C for 72 h. After the reaction, a brown–red floccule was obtained. After that, the floccule was collected, washed with distilled water and ethanol several times to remove ions and possible remnants in the final product.Characterization
The final products were characterized by various techniques. X-Ray powder diffraction (XRD) was carried out on a Rigaku D/max-rA X-ray diffractometer with Cu Kα radiation (λ = 1.54178 Å). The scan rate of 0.05° s−1 was applied to record the pattern in the 2θ range of 40–90°. The morphology and size of as-prepared products were observed by field emission scanning electron microscope (FESEM) and transmission electron microscope (TEM) images, which were taken on a Hitachi model H-800 and performed on a JSM-6700F field emission scanning electron microanalyzer, respectively. The high-resolution TEM images (HRTEM) and the corresponding selected-area electron diffraction (SAED) patterns were taken on a JEOL 2010 high-resolution transmission electron microscope performed at an acceleration voltage of 200 kV. To obtain further evidence for the purities and compositions of the as-prepared products, the X-ray photoelectron spectra (XPS) were used, which were recorded on an ESCALab MKII X-ray photo-electron spectrometer, using Mg Kα radiation as the exciting source. IR spectra were measured on a Bruker Vector-22 FT-IR spectrometer at room temperature.Results and discussion
Synthesis of Cu@cross-linked PVA nanocables
Fig. 1 shows the X-ray diffraction patterns of the product obtained at 200 °C for 72 h. All the peaks can be readily indexed as face-centered cubic copper with a calculated lattice constant a = 3.614 Å, which is in good agreement with the reported data (JCPDS 4-836, a = 3.615 Å). | ||
| Fig. 1 XRD pattern of the product obtained at 200 °C for 72 h. | ||
A general overview FESEM image (Fig. 2a) demonstrated that the final product consists of a large quantity of flexible fibers with a diameter of 150–500 nm and length up to 100 µm. A zoom-in image in Fig. 2b clearly shows that fibrous like structures are formed. In addition, a lot of near-spherical and non-spherical aggregates with a size of 1–3 µm are also observed (Fig. 2).
 | ||
| Fig. 2 SEM images of the product obtained at 200 °C for 72 h. | ||
The TEM image (Fig. 3a) indicates that the nanofibers are in fact nanocables, and each fiber is composed of a nanowire core about 80 nm in average diameter and a surrounding sheath about 200 nm in average thickness. The structural characterization of an individual nanocable was investigated in detail by HRTEM and selected area electron diffraction (SAED) (Fig. 4). The HRTEM images (Fig. 4b,c) of the nanocables clearly demonstrated that the as-obtained Cu nanowires are coated with cross-linked PVA layers and the core Cu nanowires grow preferentially along the [110] direction with a lattice spacing of 2.58 Å, which is different from the previous report for bare copper nanowires synthesized from solution.13 The SAED pattern recorded from the core section of the nanocable clearly indicates that the diffraction indexes are in complete agreement with that for Cu (fcc). Previously, it has been reported that there is a thin Cu2O oxide layes on the outside of the Cu nanowires.14 However, here, the local HRTEM image indicates that there is no Cu2O oxide layers (Fig. 4b), revealing that PVA protects copper nanowires from oxidation.
 | ||
| Fig. 3 (a), (b) TEM images of copper/cross-linked nanocables obtained at 200 °C for 72 h; (c), (d) show typical nanocables with a single wire as core and two parallel wires as core. | ||
![(a) TEM image of a thinner copper nanocable with a diameter of 50 nm and a thinner sheath of 10 nm, the sample was obtained at 200 °C for 72 h. (b), (c) HRTEM images taken on the areas marked in (a), showing that the fringe spacings are 2.58 Å, corresponding to the interplanar distance of (110) planes, indicating that the growth direction of this nanocable was [110]. (d) SAED pattern shows the wire is single crystalline.](/image/article/2006/JM/b511721f/b511721f-f4.gif) | ||
| Fig. 4 (a) TEM image of a thinner copper nanocable with a diameter of 50 nm and a thinner sheath of 10 nm, the sample was obtained at 200 °C for 72 h. (b), (c) HRTEM images taken on the areas marked in (a), showing that the fringe spacings are 2.58 Å, corresponding to the interplanar distance of (110) planes, indicating that the growth direction of this nanocable was [110]. (d) SAED pattern shows the wire is single crystalline. | ||
The XPS spectrum in Fig. 5 indicated that C1s and O1s binding energies of the obtained sample are 284.67 and 532.40 eV, respectively. However, the binding energy at 933.90 eV for Cu 2p1/2 almost cannot be detected. The quantitative analysis of the sample indicates that the molar contents of C, O, Cu are, respectively, 80.33%, 18.65% and 1.02%. Therefore, the XPS analysis confirmed that all copper nanowires are confined within shells of the cross-linked PVA, considering that the XPS is a very surface-sensitive analytical technique.
 | ||
| Fig. 5 XPS spectrum of the Cu/PVA nanocables obtained at 200 °C for 72 hours. | ||
Phase and shape evolution
It has been found that the formation of the obtained nanocables is strongly dependent on the reaction time. The time dependent shape evolution of the structures was done by examining the products obtained after different reaction times (Fig. 6). After 12 h, a large amount of aggregate was generated (Fig. 6a). After the reaction for 36 h, some nanocables entangled with the surface of the sphere-like aggregates can be found (Fig. 6b). When the reaction was prolonged up to 72 h, relatively complete copper/cross-linked PVA cables were obtained (Fig. 6c), accompanying some aggregates. At the same time, the XRD pattern (Fig. 7a,b) indicates that the CuCl phase is an intermediate product during the formation of the Cu phase. CuCl phase is formed in the early stage and then converted to Cu/cross-linked PVA nanocables in later stage. This can be explained by the fact that the reducing process of Cu2+ involves two distinct steps, which demonstrated that longer reaction time is necessary for the formation of Cu/cross-linked PVA nanocables. Compared with the uniform silver/cross-linked PVA nanocables we achieved previously, the presence of a large amount of cross-linked PVA aggregates here could be due to the fact that the cross-linking reaction in the presence of different metal ions has different reaction rates, and thus could determine the uniformity of the product as well as the quality of the cable-like core–shell structures.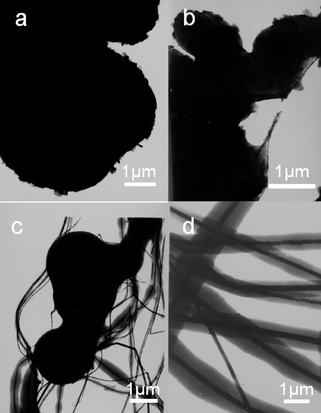 | ||
| Fig. 6 (a–d) TEM images of the shape evolution of products after reaction at 200 °C (pH = 11.3) for different periods: (a) 6 h, (b)12 h, (c) 36 h, (d) 72 h. | ||
 | ||
| Fig. 7 XRD patterns of the products obtained under different conditions: (a) 200 °C, 12 hours, (b) 200 °C, 36 hours and (c) 180 °C, 72 hours. # CuCl phase, * Cu phase. | ||
It should be noted that the temperature also has a strong influence on the formation of the cables. The default experiments show that the CuCl particles were generated if the similar reaction was done at 160 °C even for 3 days, and no Cu@cross-linked PVA cable-like structures can be obtained. Compared with that for the formation of silver@cross-linked PVA cables, higher temperature is required for the formation of copper@cross-linked PVA cables.12 The reaction of CuCl2 with PVA at 180 °C under similar hydrothermal conditions leads to the formation of only a small number of nanocables (Fig. 8b) with not well-defined structures. The XRD pattern indicated that the sample obtained at 180 °C (pH 11.3, 72 h) contains a small amount of CuCl impurity (Fig. 7c).
 | ||
| Fig. 8 TEM images showing differences of the growth of copper/PVA nanocables for 72 hours. (a) 160 °C, starting pH 11.3; (b)180 °C, starting pH 11.3. | ||
Furthermore, the suitable initial pH value of the reaction solution is also essential for the formation of cables. The contrast experiments, keeping the initial pH value in a range of 7.0–9.0, indicated that PVA cannot be cross-linked, even under identical conditions. The pH value dropped dramatically from the initial value of 7.0–9.0 to 1.4–2.0 for the residual solution after the reaction for 72 hours, implying that the solution after reaction becomes more acidic. When the initial pH value was kept as acidic, no reaction can happen. Furthermore, the reaction process can result in the existence of abundant H+ in the solution, which possibly makes the newly-produced copper dissolve. Therefore, a suitable pH value as well as a suitable reaction time are required in order to keep the stability of the copper nanowires. It was found that the nanocables can be obtained when the initial pH value of the reaction solution was controlled within a range from 10 to 13. The pH value dropped dramatically from 10–13 for the starting solution to 4.5–5.2 for the residual solution after reaction.
Reaction mechanism
Both the possible reaction mechanism and the formation mechanism of cables are proposed. It is well known that PVA chains will become cross-linked after treatment at high temperature (250 °C),9 however, we find that it is difficult to make PVA solution cross-linked via hydrothermal treatment at 160 °C or even 200 °C in the absence of any ions. Remarkably the cross-linked reaction that occurred at relatively low temperature under the closed hydrothermal system conditions could be due to the presence of Cu2+. The contrast experiments in the absence of CuCl2 show that PVA cannot be cross-linked, even under identical conditions. In addition, the rapid reduction of CuCl2 into Cu nanoparticles in the same solution with an identical concentration of PVA and then underwent hydrothermal treatment at the same temperature for the same reaction period cannot produce such cables. These results demonstrated that the presence of Cu2+ is essential for the formation of cables, and Cu2+ indeed plays a key role as a similar effect as we reported previously in the case of silver ions, i.e., acting as a catalyst for oxidation–reduction reactions occurring in this system.12Based on the XRD results of Fig. 7a,b, it can be concluded that the oxidation–reduction reaction process of Cu2+ involves two distinct steps. Namely, Cu2+ is first reduced by PVA to CuCl, then CuCl is reduced by PVA to Cu. With the prolongation of the time, the combination of OH−and H+ promotes the reactions to complete and finally produces the Cu phase. The reducing process of Cu2+ into Cu could be also similar to that well-known phenomenon of the formation of metal nanoparticles like Ag, and Pd at elevated temperature by the so-called polyol process where the radicals were generated and acted as a reducing agent.15
In the meantime, in this process, PVA can be oxidized by Cu2+ at 200 °C into cross-linked PVA (insoluble in water with a brown red color). The whole reaction process under the present hydrothermal conditions can be described as follows:
The reaction mechanism can be further testified via the FTIR spectrum of the obtained nanocables, which is almost the same as that of pure PVA. Fig. 9 shows that both spectra have characteristic C–H stretches at 2914 cm−1 and 2820 cm−1, O–H stretches at 3428 cm−1 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretches at 1735 cm−1. However, there is a stronger absorption peak from 1060 to 1100 cm−1, which can be assigned as the absorption peak of υ(C–O–C) of the cross-linked PVA. Similarly, there may be some ester in the product due to the presence of a peak at 1735 cm−1 as we found in the case of silver/PVA nanocables.12 In fact, we have proposed that the formation of such Ag/cross-linked PVA nanocables is controlled by a so-called synergistic soft–hard template mechanism (SSHM).12 Herein, PVA is again responsible for both the formation of copper nanoparticles and further their oriented growth into copper nanowires stabilized by PVA and their cross-linked component; in turn, the copper wires act as a backbone on which cross-linked PVA will form.12
O stretches at 1735 cm−1. However, there is a stronger absorption peak from 1060 to 1100 cm−1, which can be assigned as the absorption peak of υ(C–O–C) of the cross-linked PVA. Similarly, there may be some ester in the product due to the presence of a peak at 1735 cm−1 as we found in the case of silver/PVA nanocables.12 In fact, we have proposed that the formation of such Ag/cross-linked PVA nanocables is controlled by a so-called synergistic soft–hard template mechanism (SSHM).12 Herein, PVA is again responsible for both the formation of copper nanoparticles and further their oriented growth into copper nanowires stabilized by PVA and their cross-linked component; in turn, the copper wires act as a backbone on which cross-linked PVA will form.12
 | ||
| Fig. 9 FTIR spectra: (a) PVA, (b) Cu/cross-linked PVA nanocables obtained after reaction at 200 °C for 72 h. | ||
Conclusions
In summary, large-scale synthesis of flexible Cu@cross-linked PVA nanocables can be achieved by a hydrothermal approach at 200 °C. In contrast to our previous findings in the case of silver ions, metal ions with high valency are more difficult to reduce during the cross-linking reactions under hydrothermal conditions. Both the initial pH value and the reaction temperature have significant effects on the reaction and the quality of the product. Copper@cross-linked PVA nanocables with a diameter of 0.5–1 µm and length up to 100 µm can be produced at 200 °C. Besides the formation of nanocables, the cross-linked PVA aggregates with near spherical shape and irregular shapes have also been observed, underlining that the cross-linking reaction in the presence of different metal ions has different reaction rates, resulting in the differences in the uniformity of the product and the quality of the cable-like core–shell structures. Further optimization of this reaction could avoid the presence of such cross-linked PVA aggregates to access uniform nanocables with high quality. The present study demonstrated that other high valent metal ions could be also reduced by this similar approach, making it possible produce other cable-like structures. The cables with noble metal as core are expected to find applications as conducting wires for the connection of minitype devices and in other fields.Acknowledgements
This work was supported by the special funding support from the Century Program of the Chinese Academy of Sciences, the Natural Science Foundation of China (Contract No. 20325104, 20321101, 50372065).References
- (a) C. M. Lieber, Sci. Am., 2001, 285, 58 CrossRef CAS; (b) J. Hu, T. W. Odom and C. M. Lieber, Acc. Chem. Res., 1999, 32, 435 CrossRef CAS.
- Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim and H. Yan, Adv. Mater., 2003, 15, 353 CrossRef CAS.
- (a) Z. L. Wang, J. Mater. Chem., 2005, 15, 1021 RSC; (b) Z. L. Wang, X. Y. Kong, Y. Ding, P. X. Gao, W. L. Hughes, R. S. Yang and Y. Zhang, Adv. Funct. Mater., 2004, 14, 943 CrossRef CAS; (c) X. S. Fang, C. H. Ye, X. S. Peng, Y. H. Wang, Y. C. Wu and L. D. Zhang, J. Mater. Chem., 2003, 13, 3040 RSC.
- G. M. Whitesides, Small, 2005, 1, 172 Search PubMed.
- (a) F. Caruso, Adv. Mater., 2001, 13, 11 CrossRef CAS; (b) K. J. C. Van Bommel, A. Friggeri and S. Shinkai, Angew. Chem., Int. Ed., 2003, 42, 3027 CrossRef CAS.
- (a) A. M. Morales and C. M. Lieber, Science, 1998, 279, 208 CrossRef CAS; (b) W. S. Shi, H. Y. Peng, L. Xu, N. Wang, Y. H. H. Tang and S. T. Lee, Adv. Mater., 2000, 12, 1927 CrossRef CAS.
- Y. Zhang, K. Suenaga, C. Colliex and S. Iijima, Science, 1998, 281, 973 CrossRef CAS.
- G. W. Meng, L. D. Zhang, C. M. Mo, S. Y. Zhang, Y. Qin, S. P. Feng and H. J. Li, J. Mater. Res., 1998, 13, 2533 CrossRef CAS.
- Y. Xie, Z. Qiao, M. Chen, X. Liu and Y. Qian, Adv. Mater., 1999, 11, 1512 CrossRef CAS.
- Y. Yin, Y. Lu, Y. Sun and Y. Xia, Nano Lett., 2002, 2, 427 CrossRef CAS.
- C. F. Monson and A. T. Woolley, Nano Lett., 2003, 3, 359 CrossRef CAS.
- L. B. Luo, S. H. Yu, H. S. Qian and T. Zhou, J. Am. Chem. Soc., 2005, 127, 2822 CrossRef CAS.
- S. H. Yu, X. J. Cui, L. Li, K. Li, B. Yu, M. Antonietti and H. Cölfen, Adv. Mater., 2004, 16, 1636 CrossRef CAS.
- Z. P. Liu, Y. Yang, J. P. Liang, Z. K. Hu, S. Li, S. Peng and Y. T. Qian, J. Phys. Chem. B, 2003, 107, 12658 CrossRef CAS.
- Y. Sun and Y. Xia, Adv. Mater., 2002, 14, 833 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2006 |


