Rapid breakdown of brominated flame retardants by soil microorganisms
Anne P.
Vonderheide
*a,
Sabrina R.
Mueller-Spitz
b,
Juris
Meija
ce,
Gwendolyn L.
Welsh
a,
Kevin E.
Mueller
d,
Brian K.
Kinkle
a,
Jodi R.
Shann
a and
Joseph A.
Caruso
e
aDepartment of Biological Sciences, University of Cincinnati, Cincinnati, Ohio 45221-0006, USA. E-mail: pawleca@email.uc.edu
bGreat Lakes WATER Institute, University of Wisconsin-Milwaukee, Milwaukee, Wisconsin 53204, USA
cInstitute for National Measurement Standards, National Research Council Canada, Ottawa, Ontario, Canada K1A 0R6
dIntercollege Program in Ecology, Penn State University, University Park, Pennsylvania, 16802, USA
eDepartment of Chemistry, University of Cincinnati, Cincinnati, Ohio 45221-0172, USA
First published on 20th September 2006
Abstract
Polybrominated diphenyl ethers (PBDEs) have been extensively and successfully used as fire retardants in a multitude of products. However, due to their aromatic components and toxicological properties, they are assumed to be persistent environmental pollutants and a popularly-used commercial mixture, DE-71, has been removed from American and European marketplaces. However, our current work shows mixed bacterial cultures, derived or extracted from soils experimentally contaminated with DE-71, were capable of utilizing these as a sole carbon source. Most notably, almost complete loss of parent compounds takes place within a few minutes. Determining pathways was hindered by both the speed of the microbial degradation and the low water solubility of the congeners, complicating detection. The bacterial enrichment communities have been characterized using DGGE analysis and DNA sequencing. Element-specific detection was coupled to ion chromatography and only one degradation product detectable by ICP-MS, the bromide ion, was found. Additionally, samples were analyzed by GC/TOF-MS using a mass-defect-based digital noise filtering technique to facilitate observation of bromine-containing unknowns and ESI-MS was used to identify the non-volatile brominated unknown peaks. However, low concentrations (100 μg L−1 initial fortification and substantially lower levels of theorized by-products) prohibited successful compound identification.
Introduction
Polybrominated diphenyl ethers (PBDEs) are a class of compounds incorporated into numerous commercial goods at production facilities around the world. Their purpose is to reduce flammability and as a result of their extensive application and additive incorporation (no covalent bonding), subsequent release during product use and disposal has resulted in increasing levels of these compounds in biota and environmental compartments.1,2 Three commercial mixtures are sold, each differing in degree of bromination. The penta-BDE mixture (DE-71) was banned in Europe3 and the US Congress subsequently asked American manufacturers to phase out production of this mixture.4 This commercial formulation consists of tetra-, penta- and hexa-brominated diphenyl ethers and is of particular concern as the concentration of its congeners is on the rise in matrices investigated.5–8PBDEs enter the environment in a different manner than many other persistent organic pollutants (POPs), which generally infiltrate the environment directly from point sources. The PBDEs, in contrast, are released from products over their entire lifetimes, not only as point source pollution during manufacture.9 Environmental occurrence of the lower-brominated congeners is also attributed to the biological10 and photolytic11,12 breakdown of the still-used decabrominated BDE commercial mixture.13–16 At present, it is unclear what proportion of the tetra- to hexa-BDEs found in the environment are breakdown products of the deca-BDE congener and what proportion originates from commercial penta-BDE mixtures.
Bioaccumulation in humans is attributed to the persistence and lipophilicity of the congeners. Studies conducted on human adipose tissues, serum and milk demonstrate increasing concentrations of PBDEs.5,17,18 Intake is primarily ascribed to diet and indoor atmospheres.19 Elevated concentrations in humans are of growing concern because other animal studies indicate that certain congeners can cause neurotoxic effects, interfere with brain development, bind to the thyroid and aryl hydrocarbon receptor, and disrupt endocrine properties.20,21
Presently, scientific opinion suggests that these compounds will accumulate in the environment and their fate is attributed to structural stability and subsequently limited degradation.22,23 However, knowledge of PBDE metabolism is restricted primarily to experimental studies in higher animals. The ability of soil microbes to degrade halogenated POPs24–26 is an important component of determining the fate of organic contaminants in the environment. Previous research has shown that mono- and di-BDEs are subject to aerobic attack by Sphingomonas sp., however only the mono-BDE was utilized as a carbon source and the stereochemistry of higher BDEs was suggested as a limiting factor for aerobic degradation.27,28 More recently, single debromination by anaerobic bacteria of BDE-15 (di-BDE)29 and BDE-209 (deca-BDE)30 has been measured.
The purpose of this work was to determine the degradative ability of bacterial enrichment consortia against the penta PBDE commercial mixture in aqueous solutions. Supporting microbial data are presented along with DNA characterization of the community present. In the area of chemical analysis, the extreme hydrophobic nature and subsequent low concentrations utilized would challenge even the most established and reliable instrumental techniques; a complement of atomic (ICP-MS) and molecular spectrometry (TOF-MS and ESI-MS) was employed to investigate the ability of soil microbial enrichment cultures to utilize penta-BDEs as a carbon source. Vonderheide et al. established the feasibility of using ICP-MS in the detection of these brominated contaminants.31 GC/TOF-MS is a very common molecular mass spectrometric technique and is highly useful when used in conjunction with ICP-MS because of their comparable detection limits. Further, Focant et al. employed TOF-MS for detection of selected PBDEs32 and Kazda et al. demonstrated detection limits in the low ppb range with negative chemical ionization using the bromine atom (m/z = −80.916) for quantification.33
Experimental
Reagents and standards
All water was deionized (18 MΩ cm) and prepared by passing through a NanoPure treatment system (Barnstead, Boston, MA). Commercial chemicals were of analytical reagent grade and were used without further purification. Analytical standards were purchased from AccuStandard (New Haven, CT). These included 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47), 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99), 2,2′,4,4′,6-pentabromodiphenyl ether (BDE-100). Solvents used for standard preparation (hexane and acetone) were purchased from Fisher (Fairlawn, NJ).Ciprofloxacin was purchased from Sigma (St. Louis, MO).
Instrumentation
Characterization of the penta-BDE commercial mixture
The penta-BDE commercial mixture, DE-71, is produced by the Great Lakes Chemical Company. Composition is detailed in Table 1. The column labeled “Most abundant monoisotopic mass” contains, for example, the mass of C12H8O79Br81Br for a dibrominated diphenyl ether.| Congener | Molecular formula | Most abundant monoisotopic mass/Da | Compound name |
|
|---|---|---|---|---|
| BDE-47 | C12H6Br4O | 485.711 | 2,2′,4,4′-Tetrabromodiphenyl ether | x = 2, y = 2 |
| BDE-99 | C12H5Br5O | 563.622 | 2,2′,4,4′,5-Pentabromodiphenyl ether | x = 3, y = 2 |
| BDE-100 | C12H5Br5O | 563.622 | 2,2′,4,4′,6-Pentabromodiphenyl ether | x = 3, y = 2 |
| BDE-85 | C12H5Br5O | 563.622 | 2,2′,3,4,4′-Pentabromodiphenyl ether | x = 3, y = 2 |
| BDE-154 | C12H4Br6O | 643.530 | 2,2′,4,4′,5,5′-Hexabromodiphenyl ether | x = 3, y = 3 |
| BDE-155 | C12H4Br6O | 643.530 | 2,2′,4,4′,5,6′-Hexabromodiphenyl ether | x = 3, y = 3 |
The portion of DE-71 used in this study was donated by Dr Raoul V. Kuiper (Department of Pathobiology, Faculty of Veterinary Medicine, Utrecht University, Netherlands). Initial characterization was performed through analysis of a highly concentrated solution of the DE-71 under full scan mode on an Agilent GC-MS system. One tetra-brominated (BDE-47), three penta-brominated (BDE-85, 99 and 100) and two hexa-brominated (BDE-153 and 154) congeners were found to be present in the commercial mixture. The ratio of the peak areas of the chromatogram were used to approximate the ratio of their concentrations. The amount fraction of each congener was determined to be as follows: BDE-47: 46.0%; BDE-100: 12.6%; BDE-99: 36.7%; BDE-85: 1.3%; BDE-154: 2.1% and BDE-153: 1.3%.
Soil properties and preparation
A silty clay loam (19% sand, 53% silt and 27% clay) with a water holding capacity of 50% was used for this study. The soil had the following nutrient levels: 29.8 mg kg−1 NO3− (as N), 2.5 mg kg−1 NH4+ (as N), 52.5 mg kg−1 PO43−, 6720 mg kg−1 Ca2+ and 417 mg kg−1 Mg2+. Soil contained 1.8% organic matter and its pH was 7.7. Cation exchange capacity was 17.3 cmol cations kg−1. All soil properties were determined by the Agricultural Analytical Services Laboratory of Pennsylvania State University. Prior to PBDE-amendment, the pH of the loam was adjusted to 6.8. A small aliquot of soil was spiked with the DE-71 penta-brominated commercial mixture (delivered in acetone), mixed thoroughly, and placed under a fume hood for solvent evaporation. Spiked soil was then continuously tumbled with un-spiked soil for 2 h at room temperature to ensure efficient mixing and bring the soil to a final concentration of 75 μg kg−1, chosen to best mimic environmental levels determined to date.Planting
In order to establish different microbial communities for investigation, different plant species were grown in the DE-71 contaminated soil. Further, an unplanted treatment was maintained to differentiate between communities exposed to plant root exudates and those without such an input. Radish (Hybrid radish, small round red cherriette F1; Raphanus sativus) and summer squash (Hybrid zucchini squash, yellow gold rush F1; Cucurbita species) seeds were purchased from Johnny Seeds (Winslow, Maine). Pots either contained one zucchini plant or four radish plants or were left unplanted. Further, mixed pots contained both one zucchini and four radish plants. Pots were maintained in a controlled growth room (15/20 °C, 8 h dark/16 h light, 520 lux) for 10 weeks and were daily brought up to 75% of the water holding capacity.Microbial analysis
After plant harvest, microorganisms were isolated from the experimentally contaminated soil of each planting treatment (n = 4) by using a selective enrichment following the procedure detailed by Focht.34 Briefly, a 5 g sample of contaminated soil was combined with 100 mL of a mineral salts media supplemented with yeast extract (25 mg L−1) and 500 μg L−1 of the DE-71 mixture in acetone. The yeast extract was included in the enrichments to provide a source of cofactors for organisms not able to synthesize their own. The soil slurry was incubated at 20 °C on a rotary shaker. After seven days, 0.5 mL of the enrichment was transferred to 50 mL of fresh mineral salts media containing yeast extract and PBDEs. After another five days, the enrichment cultures were transferred again to fresh media and this was repeated two times. The mixed bacterial cultures were used for all subsequent analyses.Community analysis of enrichment cultures
Cells from each enrichment culture were used for DNA isolation following a modified procedure from Boom et al.36 PCR of the DNA was carried out using primers that target the V3 region of the 16s rRNA gene of using the primers F357-GC (5′-GC-clamp-GCCTACGGAGGCAGCAG-3′) and 518R (5′-ATTACCGCGGCTGCTGG-3′) yielding a 194 base-pair fragment.37 PCR was performed using the MasterTaq Kit (Eppendorf AG, Germany). The reactions were carried out in a total volume of 50 μL with 5 μL TaqMaster Enhancer for bacterial amplification and 10 μL of the TaqMaster Enhancer for fungal amplification, 5 μL 10X buffer, 1.5 μL of V3 primers (10 μM of each primer), 1.5 μL dNTPs (10 μM of each nucleotide), 0.3 μL Taq Polymerase (5 U μL−1), 34.7 μL water, and 2 μL of template DNA. The following PCR program was followed to amplify the V3 region: denaturation at 94 °C 5 min; 35 cycles of 94 °C for 45 s, annealing 56 °C for 45 s, and extension 68 °C for 1 min; and final extension for 7 min at 68 °C, followed by cooling at 4 °C. Bacterial PCR products were analyzed by denaturing gradient gel electrophoresis (DGGE). Gels were cast using a 7.5% polyacrylamide solution with denaturing gradients from 35 to 60% (100% denaturant corresponds to 7 M urea and 40% [v/v] of deionized formamide). The gels were run at 200 V for 1 h and 150 V for 4 h at 60 °C for 16 × 16 cm gels or 200 V for 1 h and 150 V for 5 h for 16 × 20 cm gels. The short run time and high voltage was used to ensure all bands would be detected on the gel. Gels were stained for 30 min with a 1 : 5000 dilution of SBYR Green (Molecular Probes; Eugene, OR). After staining, gels were viewed using a gel documentation system (NucleoTech; San Mateo, CA). Images of the DGGE gels were imported into Bionumerics v. 3.0 (Applied Maths, Kortrijk, Belgium) for analysis of the banding patterns. Enrichment communities were compared using a densitometric curve-based method that evaluates the intensity as well as the position of the bands to generate pairwise similarity scores (Pearson coefficient). Individual DGGE bands were isolated from the polyacrylamide gel and soaked in sterile water and subjected to PCR under the conditions previously stated. PCR products were cleaned for sequencing using QIAquick PCR Purification kit (Qiagen, Valencia, CA.). Cleaned PCR products were sequenced on ABI PRISM® 3730 DNA Analyzer (Applied Biosystems, Foster City, CA) at Cincinnati Childern’s Hospital Medical Center (Cincinnati, OH). Sequences were compared to the RDP-II database using Seqmatch v.3 and the NCBI database using a blast search to determine the genus-level classification of organism represented by the DGGE bands.Degradation experiment
A time course study was carried out to determine the rate of loss of the DE-71 mixture. After the enrichment consortia from each planting treatment were washed to remove any PBDEs from the growth media, cells were combined with 100 μg L−1 of DE-71 and minimal salts media.34 Samples were removed at 2, 24 and 48 h for GC/TOF-MS analysis. The experiment was repeated with sampling begun immediately after addition of the PBDEs at 5, 15, 30, 60, 90 and 120 min. Samples were immediately extracted or acidified (depending on subsequent analytical procedure) to prevent further congener modification or loss.Analytical sample preparation
For GC analysis, water samples were extracted with hexane (1 : 1) but were not subjected to preconcentration. For analysis by ESI, water samples were acidified with formic acid. For HPLC-ICP-MS analysis, water samples were diluted 1 : 1 in the mobile phase.Results and discussion
Microbial analysis
The hydrophobicity of these compounds is well documented and increasing molecular weight by sequential bromine atom addition only exacerbates the low solubility. Although not all PBDE congeners give documented numbers, compounds with more than eight bromine atoms are listed with a water solubility of less than 0.1 μg L−1.38 We initially investigated the viability of the experimental setup to assure that any loss of PBDE was due to microbial action. Plastic containers previously planned for use were found to adsorb PBDEs; hence, only glass apparatus was employed throughout. Additionally, concern existed regarding the potential for these compounds to adsorb onto the cell walls of the microorganisms. A trial employing the use of dead bacterial cells, however, found this was of no consequence (data not shown).Initial experiments investigated the microbial degradation potential of the penta-BDEs. Direct viable counts provided preliminary evidence for microbial utilization of PBDEs. Elongation of microbial cells grown using the PBDEs as their sole carbon source was observed in the presence of the antibiotic ciprofloxacin, which inhibits cell division. Approximately 2–50% of the bacteria in the enrichment cultures were able to grow and replicate in the presence of 100 μg L−1 DE-71 and 25 μg L−1 yeast extract (see Fig. 1 for the enrichment consortia grown from unplanted contaminated soil). Elongated cells were also observed in treatments where penta-BDE was the only carbon source provided. The zucchini monoculture treatment did not show an increase in elongated cells when PBDEs were present, suggesting that either the concentration of ciprofloxacin was not effective on the members of the consortia based on the number of small cells present or the cells were not actively growing and subsequently undergoing division. The unplanted treatment had the greatest number of elongated cells (see Fig. 1), perhaps suggesting that without carbon input from plant roots, the soil microbial community was better adapted to metabolize penta-BDEs. In conclusion, this consortium contained individuals capable of metabolizing PBDEs in solution.
 | ||
| Fig. 1 Cell elongation indicates the ability to utilize a given carbon source as a growth substrate. Graph shows amount of elongated cells in enrichment consortia grown from unplanted contaminated soil after 96 h in the presence of ciprofloxacin, yeast extract and/or DE-71 at 100 μg L−1. Part b demonstrates the cell elongation observed under specified conditions, as opposed to part a. | ||
Chemical analysis
Initial experiments established microbial exposure to the PBDEs for a period of 2 to 48 h. Aqueous samples were extracted with hexane but not subjected to preconcentration and so extremely selective mass spectral detection (GC/TOF-MS) was employed. Additionally, the exact mass capability of this instrument was an attractive feature and this characteristic was further exploited as discussed later. Others have investigated the potential of this instrument for the analysis of the brominated flame retardants in negative CI mode.33 Stapleton notes this yields little spectral advantage as bromide ions are found in greatest abundance as opposed to molecular fragments.39 Others used electron impact; in our work, positive chemical ionization was additionally explored.Due to low levels expected, a simple yet discernable molecular ion and fragmentation pattern were sought. A comparison of the fragmentation of BDE-99 under each of the ionization modes is shown in Fig. 2. In electron impact (EI) mode, only neutral loss Br2 is observed (about 50% fragmentation). There is no H transfer and ion clusters possess purely binomial distributions. Use of positive chemical ionization demonstrates multiple losses of Br radicals as well as the formation of hydrogen adducts. Finally, with negative chemical ionization, 100% loss of the Br radical is observed. Again there is no H transfer and ion clusters possess a purely binomial distribution. EI ionization was chosen for its simplicity of spectra (vs. positive chemical ionization) and its confirmation of the molecular ion (vs. negative chemical ionization). Spectra for the tetra-, penta- and hexa-brominated congeners are given in Fig. 3. Detection limits with this mode of ionization were calculated by a method based on sideband noise40,41 and were determined to be ∼5 μg L−1 for each of the three congeners.
 | ||
| Fig. 2 A comparison of the GC/TOF-MS fragmentation of BDE-99 under different ionization conditions. A concentration of 100 μg L−1 of each congener was used. Note in CI negative mode, the molecular ion is not observed, however, in CI positive mode, a sequential loss of bromine is observed. | ||
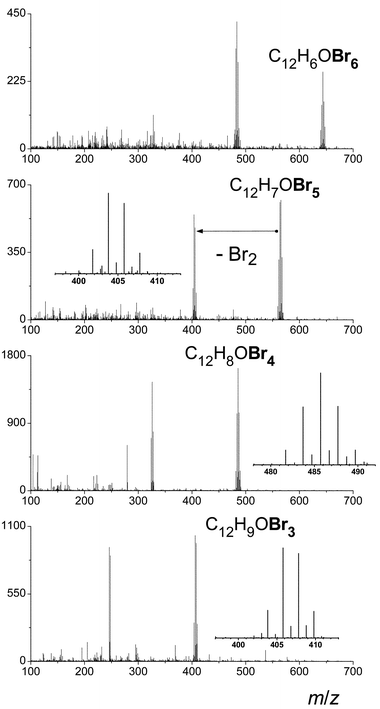 | ||
| Fig. 3 EI ionization spectra for the tetra-, penta- and hexa-brominated congeners, each at the level of 100 μg L−1. | ||
Using these established conditions, GC/TOF-MS analysis further confirmed the use of the penta-BDE mixture as a carbon source by microbial enrichment cultures. Within the shortest time period (2 h) of the initial experiment, loss of all of the DE-71 was observed (above instrumental detection limit). The time-series experiment was then repeated with shorter time intervals (5, 15, 30 60, 90 and 120 min and samples utilizing microbial enrichment cultures from planted and unplanted soils were analyzed by GC/TOF-MS at each time interval. Control samples (containing no microorganisms) were analyzed alongside those inoculated with enrichment cultures. Again, even in these shorter intervals, complete or near complete loss of the PBDEs was observed. Representative chromatograms (m/z = 486) are shown in Fig. 4 for the sample exposed to the enrichment consortia isolated from soil planted with zucchini only, radish only and the control. Areas were taken from the extracted ion chromatograms for m/z 486 (BDE-47) and m/z 566 (BDE-99).
 | ||
| Fig. 4 Chromatograms from GC-TOF-MS analysis of samples utilizing communities from planted and unplanted soils. Analysis was performed at their 5 min time points. Control samples (containing no microorganisms) were analyzed alongside those exposed to the three populations. Total y-axis offset was 7% and total x-axis offset was −6%. | ||
Investigation into the degradative pathway was initiated. The most frequently found PBDE congeners are BDE-47 (2,2′,4,4′-tetrabromodiphenyl ether), BDE-99 (2,2′,4,4′,5-pentabromodiphenyl ether) and BDE-100 (2,2′,4,4′,6-pentabromodiphenyl ether), which all share the same 2,2′,4,4′ substitution pattern.17,22,42–53 In each of the samples exposed to the three microbial enrichment consortia, BDE-99 is consumed almost instantaneously. Further, the single tetra-brominated congener underwent a similar fate, with the exception of its rather large concentration in the sample exposed to the enrichment consortia isolated from soil planted with zucchini only (see Fig. 4). Because this sample contains significantly more BDE-47 than it started with, we assume that the increased presence of the tetra-brominated species is due to degradation of a higher-brominated congener. Therefore, the first step in the degradation of the higher-brominated congener (presumably BDE-99) must consist of loss of the bromine in the 5 position.
In the search for additional degradation products, the extracted ion chromatograms of the lower-brominated congeners (mono-, di- and tri-) were constructed, however, no peaks were apparent. A mass defect-based algorithm was utilized to determine if any further bromine-containing compounds were present in the extract. A mass defect mask was applied to TOF-MS spectra and had the effect of converting electron impact mass spectra into bromine-specific mass spectra. Further, more than one bromine atom in a molecule forces the accumulation of the mass defect and reduces extraneous signals to an even greater extent. Application of this algorithm to the TOF-MS data did not reveal any other bromine-containing compounds.
It may be that after debromination to BDE-47, subsequent intermediates may be more water soluble. Debromination to this stable congener may possibly be accomplished with ease by a particular strain. It is possible that once the stable tetra-based congener is reached, degradation continues without debromination and even perhaps by a second type of microorganism. Eriksson et al. noted the photochemical reaction rate decreased with decreasing number of bromine substituents in the molecule and in some cases, it was influenced by the PBDE substitution pattern.12
Work was conducted on the aqueous phase in search of secondary remedial by-products not detected in the GC/TOF-MS analysis. The initial step was to confirm the loss of bromine atoms by searching for the presence of the bromide ion in the aqueous phase. A solution of silver ions was added to the aqueous samples from the time course study and the PBDE standard was added to a water sample to serve as a point of comparison. Precipitation was only observed in the aqueous samples where PBDEs had been exposed to the enrichment cultures. For a more definitive confirmation of degradation, the aqueous samples were further analyzed by ion chromatography coupled to ICP-MS detection. Chromatographic conditions were those established by Creed and co-workers54 and are detailed in the Experimental section. Bromine was monitored at both of its isotopes and the ratio was in accordance with the theoretical value.
Fig. 5 demonstrates some of the chromatograms resulting from this analysis. As expected, the first peak eluting in the void volume was matched to the DE-71 standard. At the end of the run, the peak eluting at approximately 8 min was determined to be the bromide ion through retention time matching. Also displayed alongside these standard chromatograms are those from the analysis of the 5 and 120 min DE-71 treatment from the microbial degradation time study for the microbial consortia isolated from the radish planted soil. The 5 min treatment showed these two peaks corresponding to the DE-71 mixture and the bromide ion as well as a peak eluting at 6 min that did not match any common bromine-containing ionic entities, such as bromate (BrO3−). The unknown molecule was not purely organic in nature based on the chromatographic mode. We suggest this peak represents a metabolite subsequent to the degradation/debromination of the BDE-47 as GC/TOF-MS showed that all PBDE congeners were consumed even as early as 5 min. ESI-MS was attempted to determine the structure of the unknown, however, detection was concentration prohibitive and no further information was obtained on the identity of this compound. The results of the 120 min treatment showed that the metabolite by-product was short-lived.
 | ||
| Fig. 5 (a) 100 μg L−1 DE-71 in water; (b) 500 μg L−1 bromide ion in water; (c) Enrichment culture from soil previously planted with radish plant exposed to DE-71 for 5 min; (d) Enrichment culture from soil previously planted with radish plant exposed to DE-71 for 2 h. The x-axis represents the area counts for 79Br. | ||
A final experiment investigated whether the use of pentaBDEs as a carbon source was an extra or intracellular process. After a 5 min exposure of the microbial communities to 100 μg L−1 DE-71, a 1 mL sample was taken and extracted with 1 mL of hexane. The aqueous phase was then sterile-filtered (0.2 μm) and an equal concentration of DE-71 was added to the sterile filtrate. After a second 5 min interval, another sample was taken and extracted. Both samples were analyzed to determine the presence of PBDEs. Comparison of the two extracts showed an equivalent loss of PBDEs, indicating an extracellular mechanism. Analysis of the filtrate by ESI-MS did not demonstrate any outstanding peaks in the high molecular weight region that might be interpreted as the responsible enzyme.
Microbial characterization
The bacterial community within each enrichment consortia was examined using DGGE for identification. The technique of DGGE separates PCR fragments of the same length using a combination of chemical denaturants to separate double stranded DNA. Denaturation of dsDNA relies on base composition where AT rich regions denature with low concentrations of chemicals as compared to GC rich regions which require higher concentrations of chemicals to separate the base-pairs. The DGGE profile for each consortium yielded between 12–15 bands (Fig. 6). Of these bands, all four enrichment consortia possessed six of the same bands. The enrichment consortia from the zucchini and zucchini/radish were 85% similar, as compared to the other consortia which were 68–75% similar.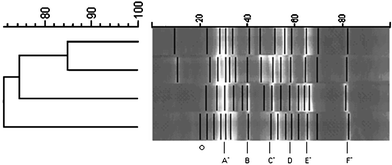 | ||
| Fig. 6 Bacterial diversity of enrichment consortia as determined by PCR-DGGE. (The left side is the similarity matrix comparing the treatments and the black lines correspond to the individual bands. Common bands are labeled at the bottom of the figure. Asterisk indicated sequence obtained from zucchini band. The top sample is zucchini/radish, next is zucchini, then unplanted, and last radish.) | ||
Bands from DGGE gels were chosen for sequencing based on relative florescence and ease of excision. Bands from zucchini were most accessible and therefore eight of its 14 bands utilized, four of which were common to the other three consortia. From the sequences obtained, four major phyla of bacteria are represented. Common band A represents both phylas Bacteroidetes and Formicates. Common bands C and E represent phyla Proteobacteria and common band F represents Actinobacteria. It was not surprising that key soil microorganisms were present in these PBDE enrichment cultures.
Conclusions
Rapid loss of PBDEs after exposure to soil microbes was demonstrated. Enrichment cultures isolated from the experimentally contaminated soils showed the ability to swiftly utilize penta-BDEs as a sole carbon source. IC-ICP-MS experiments demonstrated debromination as a partial degradative pathway. Further, absence of di- and tri-brominated congeners in the organic phase points to aqueous-soluble compounds in the final stages. It must be emphasized, however, that these experiments were performed in water and not in a soil environment. Previous research in our laboratory55 has demonstrated abiotic sorption of PBDEs to soil constituents was the most important factor in determining short term PBDE dissipation and hence, this process may not be effective in the soil environment. However, future studies are needed to evaluate the soil case.Acknowledgements
The authors would like to thank Dr Raoul V. Kuiper, Department of Pathobiology, Faculty of Veterinary Medicine, Utrecht University, Netherlands who supplied the BFRs for the project. We also acknowledge NIEHS grant #ES04908 for partial funding of this research.References
- A. Covaci, S. Voorspoels and J. d. Boer, Environ. Int., 2003, 29, 735–756 CrossRef CAS.
- R. A. Hites, Environ. Sci. Technol., 2004, 38, 945–956 CAS.
- M. Alaee, P. Arias, A. Sjodin and A. Bergman, Environ. Int., 2003, 29, 683–689 CrossRef CAS.
- R. Renner, Anal. Chem., 2005, 77, 289A–290A CAS.
- D. Meironyte, K. Noren and A. Bergman, J. Toxicol. Environ. Health, 1999, 58, 329–341 CrossRef CAS.
- M. G. Ikonomou, S. Rayne and R. F. Addison, Environ. Sci. Technol., 2002, 36, 1886–1892 CrossRef CAS.
- R. J. Norstrom, M. Simon, J. Moisey, B. Wakeford and D. V. C. Weseloh, Environ. Sci. Technol., 2002, 36, 4783–4789 CrossRef.
- B. N. Zegers, W. E. Lewis, K. Booij, R. H. Smittenberg, W. Boer, J. De Boer and J. P. Boon, Environ. Sci. Technol., 2003, 37, 3803–3807 CrossRef CAS.
- T. Hyotylainen and K. Hartonen, Trends Anal. Chem., 2002, 21, 13–29 CrossRef CAS.
- A. Kierkegaard, L. Balk, U. Tjarnlund, C. A. d. Wit and B. Jansson, Environ. Sci. Technol., 1999, 33, 1612–1617 CrossRef CAS.
- C. A. d. Wit, Chemosphere, 2002, 46, 583–624 CrossRef.
- J. Eriksson, N. Green, G. Marsh and A. Bergman, Environ. Sci. Technol., 2004, 38, 3119–3125 CrossRef CAS.
- R. C. Hale, M. J. La Guardia, E. Harvey and T. M. Mainor, Chemosphere, 2002, 46, 729–735 CrossRef CAS.
- R. E. Alcock, A. J. Sweetman, K. Prevedouros and K. C. Jones, Environ. Int., 2003, 29, 691–698 CrossRef CAS.
- C. Agrell, A. F. H. ter Schure, J. Sveder, A. Bokenstrand, P. Larsson and B. N. Zegers, Atmos. Environ., 2004, 38, 5139–5148 CrossRef CAS.
- A. F. H. ter Schure, C. Agrell, A. Bokenstrand, J. Sveder, P. Larsson and B. Zegers, Atmos. Environ., 2004, 38, 5149–5155 CrossRef CAS.
- A. Sjodin and A. Bergman, Environ. Sci. Technol., 2001, 35, 3830–3833 CrossRef CAS.
- B. Johnson-Restrepo, K. Kannan, D. P. Rapaport and B. D. Rodan, Environ. Sci. Technol., 2005, 39, 5177–5182 CrossRef CAS.
- J. L. Domingo, J. Chromatogr., A, 2004, 1054, 321–326 CrossRef CAS.
- L. S. Birnbaum and D. F. Staskal, Environ. Health Perspect., 2004, 112, 9–17 CAS.
- T. A. McDonald, Chemosphere, 2002, 46, 745–755 CrossRef CAS.
- A. Palm, I. T. Cousins, D. Mackay, M. Tysklind, C. Metcalfe and M. Alaee, Environ. Pollut., 2002, 117, 195–213 CrossRef CAS.
- T. Gouin and T. Harner, Environ. Int., 2003, 29, 717–724 CrossRef CAS.
- A. L. Juhasz and R. Naidu, J. Appl. Microbiol., 2000, 89, 642–650 CrossRef CAS.
- M. A. Manzano, J. A. Perales, D. Sales and J. M. Quiroga, Environ. Toxicol. Chem., 2003, 22, 699–705 CrossRef CAS.
- J. W. Davis, S. Gonsior, G. Marty and J. Ariano, Water Res., 2005, 39, 1075–1084 CrossRef CAS.
- S. Schmidt, P. Fortnagel and R. M. Wittich, Appl. Environ. Microbiol., 1993, 59, 3931–3933 CAS.
- S. Schmidt, R. M. Wittich, D. Erdmann, H. Wilkes, W. Francke and P. Fortnagel, Appl. Environ. Microbiol., 1992, 58, 2744–2750 CAS.
- S. Rayne, M. G. Ikonomou and M. D. Whale, Water Res., 2003, 37, 551–560 CrossRef CAS.
- A. C. Gerecke, P. C. Hartmann, N. V. Heeb, H. E. Kohler, W. Giger, P. Schmid, M. Zennegg and M. Kohler, Environ. Sci. Technol., 2005, 39, 1078–1083 CrossRef CAS.
- A. P. Vonderheide, M. Montes-Bayon and J. A. Caruso, J. Anal. At. Spectrom., 2002, 17, 1480–1485 RSC.
- J. F. Focant, A. Sjödin and D. J. Patterson, J. Chromatogr., A, 2003, 1019, 143–156 CrossRef CAS.
- R. Kazda, J. Hajšlová, J. Poustka and T. Cajka, Anal. Chim. Acta, 2004, 520, 237–243 CrossRef CAS.
- D. D. Focht, in Methods of Soil Analysis, Part 2: Microbiological and Biochemical Properties, ed. R. W. Weaver, S. Angle and P. S. Bottomly, SSSA, Madison, 1994, pp. 407–426 Search PubMed.
- P. K. Jjemba, B. K. Kinkle and J. Shann, FEMS Microbial Ecol., 2006, 287–298 Search PubMed.
- R. Boom, C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. W. V. Dillen and J. V. D. Noordaa, Clin. Microbiol., 1990, 28, 495–503 Search PubMed.
- G. Muyzer, E. C. d. Waal and A. G. Uitterlinden, Appl. Environ. Microbiol., 1993, 59, 695–700 CAS.
- S. Rayne and M. G. Ikonomou, J. Environ. Eng. Sci., 2005, 4, 369–383 Search PubMed.
- H. Stapleton, Anal. Bioanal. Chem., 2006, 1, 1–11.
- J. Meija, M. Montes-Bayon and J. A. Caruso, Anal. Chem., 2002, 74, 5837–5844 CrossRef.
- K. R. Brushwyler, N. Furata and G. M. Hieftje, Talanta, 1990, 37, 23–32 CrossRef CAS.
- J. d. Boer and W. P. Confino, Chemosphere, 2002, 46, 625–633 CrossRef CAS.
- C. Thomsen, E. Lundanes and G. Becher, J. Environ. Monit., 2001, 3, 366–370 RSC.
- C. Thomsen, E. Lundanes and G. Becher, J. Sep. Sci., 2001, 24, 282–290 CrossRef CAS.
- R. Renner, Environ. Sci. Technol., 2000, 34, 223A–226A.
- R. Renner, Environ. Sci. Technol., 2000, 34, 452A–53A CrossRef CAS.
- R. C. Hale, M. J. La Guardia, E. P. Harvey, M. O. Gaylor, T. M. Mainor and W. H. Duff, Nature, 2001, 412, 140–141 CrossRef CAS.
- J. M. Luross, M. Alaee, D. B. Sergeant, C. M. Cannon, D. M. Whittle, K. R. Solomon and D. C. G. Muir, Chemosphere, 2002, 46, 665–672 CrossRef CAS.
- R. J. Law, C. R. Allchin, M. E. Bennett, S. Morris and E. Rogan, Chemosphere, 2002, 46, 673–681 CrossRef CAS.
- C. R. Allchin, R. J. Law and S. Morris, Environ. Pollut., 1999, 105, 197–207 CrossRef CAS.
- J. K. Huwe, M. Lorentzsen, K. Thuresson and A. Bergman, Chemosphere, 2002, 46, 635–640 CrossRef CAS.
- M. G. Ikonomou, S. Rayne, M. Fischer, M. P. Fernandez and W. Cretney, Chemosphere, 2002, 46, 649–663 CrossRef CAS.
- J. She, M. Petreas, J. Winkler, P. Visita, M. McKinney and D. Kopec, Chemosphere, 2002, 46, 697–707 CrossRef CAS.
- J. T. Creed, M. Magnuson and C. A. Brockhoff, Environ. Sci. Technol., 1997, 31, 2059–2063 CrossRef CAS.
- K. Mueller, S. R. Mueller, H. F. Henry, A. P. Vonderheide, R. S. Soman, B. K. Kinkle and J. R. Shann, Environ. Sci. Technol., 2006 DOI:10.1021/es060776l.
| This journal is © The Royal Society of Chemistry 2006 |

