Green chemistry: today (and tomorrow)
James H.
Clark
Clean Technology Centre, Department of Chemistry, University of York, York, UK YO10 5DD
First published on 2nd December 2005
Abstract
It is more than 7 years since I wrote my opening article for the first issue of Green Chemistry—“Green chemistry: challenges and opportunities”.1 In this update article I will present a personal view of how the area has changed—mostly for the better—and what we need to do now. I will consider how the key drivers for major changes in the way that we practise chemistry have strengthened, how the range of relevant research has broadened, how the case studies from industry have increased and, perhaps most importantly, how our appreciation of what green chemistry should mean has matured. I will also be looking ahead at the immediate and longer-term challenges and opportunities as I now see them—in research and in industrial application and also in education and promotion.
Drivers for change
In the 1999 article1 I used the “Costs of Waste” to help provide detail on why waste was becoming increasingly expensive to industry—waste disposal, fines for pollution, loss in efficiency and costs of raw materials.These costs are even more important today and remain important drivers for change. However, we are now entering an age where legislation is likely to become as important a driver for change as process economics. When put alongside the social pressures resulting from the poor image the public have of chemical manufacturing and their largely irrational fear of “chemicals” we can now see how the three cornerstones of sustainable development—economic, environmental and social benefit, each provide the drivers of change that should help to push the application of green chemistry forward (Fig. 1).
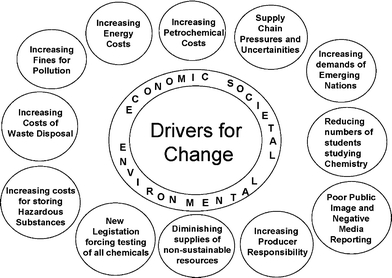 | ||
| Fig. 1 Drivers for change. | ||
What is also interesting is how these drivers now cover the three key stages of the life cycle of a chemical product—resources, manufacture and product use and fate (Fig. 2) which seems very appropriate given the essential need to see green chemistry cut across product lifecycles and help achieve true sustainability. Each stage in the lifecycle of a chemical product is resource consuming and waste generating and we cannot change one without affecting the other but for every problem there is a potential solution and with it an opportunity for commercial advantage (Fig. 2).
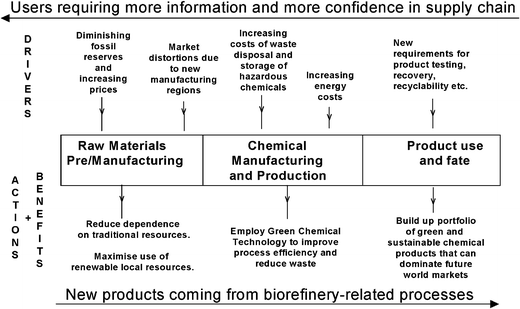 | ||
| Fig. 2 Challenges and opportunities through the chemical product lifecycle. | ||
The costs of petrochemicals—the building blocks of over 90% of today's organic chemical manufacturing have been increasing at a dramatic rate—both due to rapidly escalating oil prices and to massive market distortions due to the extraordinary growth in manufacturing industry in Asia. A good example of this is phenol, the price of which tripled in 2004 (exceeding €1 kg−1 at one point). This will force industry to reconsider its traditional feedstocks (looking more carefully at alternative sustainable sources based on biomass) and the efficiency of resource utilisation.
The costs of the disposal of hazardous substances, typically coming from the use of hazardous process auxiliaries (organic solvents, stoichiometric reagents, work-up acids and bases, etc.) and the fines for pollution climb at above the rate of inflation in many countries, while penal taxation makes the storage of large quantities of dangerous chemicals very expensive.
The new European chemicals legislation REACH—the Registration, Evaluation and Authorisation of Chemicals—is probably going to become the most important chemicals legislation we have ever seen.2
At the time of writing, the final details of the legislation are yet to be finalised and considerable pressure from the chemical industry and national governments (within and without the EU) has resulted in some significant amendments which some regard as a dilution of its power. Nonetheless, REACH will force the testing of an unprecedented number of chemicals and, apart from the added costs, REACH will undoubtedly result in a significant number of chemical applications becoming very expensive or even prohibited. While this has largely been seen as a threat to European chemical manufacturers by industry, I believe that it can also be seen as an opportunity. By being forced to test chemicals, employ more benign substitutes where necessary and build up detailed information about toxicology and environmental impact, European manufacturers will be able to claim an unmatched level of ‘green credentials’ for their products which should give them an edge in world markets where consumers are becoming increasingly concerned about chemicals. It was interesting to hear recently from a European Trades Union representative that some countries outside of Europe are viewing REACH as protectionist and threatening to their industries rather than it giving them a cost advantage in the European market.3 Alongside REACH, the EU is also introducing more product or sector related chemicals legislation such as the Restriction on Hazardous Substances (RoHS), which will prohibit or severely restrict the use of the most dangerous chemicals in electronic and electrical equipment. This has already resulted in some companies advertising RoHS compliant products ahead of the legislation and thus attempting to secure a market lead based on greener products. I believe that other regions in the world will follow the EU trend and impose ever more challenging and restrictive chemicals legislation encouraged by public and media pressure to match that in other regions. In the USA for example, new legislation will more carefully control the chemical substances used in items handled by children.
As the economic opportunities for greener products becomes more apparent so should this process of product greening accelerate and in an ideal situation, iterative innovation to improve the environmental performance of chemical products will become continuous and embedded in industry's philosophy. Now is the time as the drivers come into effect to ensure that this process is indeed sustainable by never losing sight of the lifecycle of any chemical product.
Progress in research into green and sustainable chemistry
The last 6 years has seen a significant growth in the volume and scope of green chemistry related research. This has been driven by numerous national and, to a lesser extent, trans-national initiatives to fund the area and by the growing appreciation of the value of green chemistry at all stages in the lifecycle from “cradle to grave” (Fig. 3). It is pleasing to see that sustainable chemistry will feature in “Framework 7”—the new EU research funding programme that will come into effect in 2006.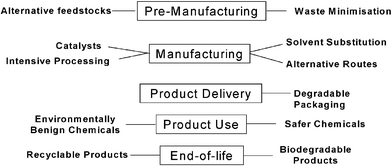 | ||
| Fig. 3 Green chemistry applied from the cradle to the grave. | ||
While the bulk of the research effort continues to be in the manufacturing box (with catalysis, alternative solvents and new routes continuing to dominate) reports of research at all stages in the lifecycle are emerging and with this journal seeing publications in many areas (Fig. 4).
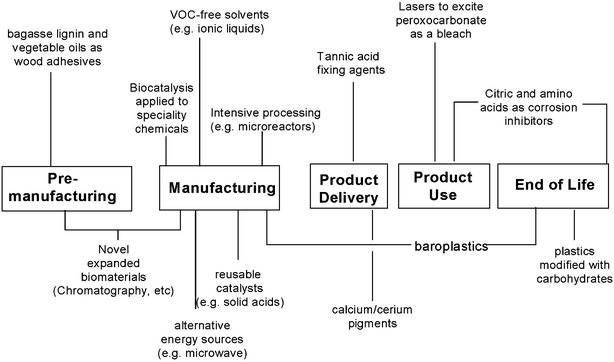 | ||
| Fig. 4 Some examples of recent progress in green chemistry R&D. | ||
I am, however, concerned that while renewable resource-based research is gaining momentum largely driven by the White Biotechnology revolution,4 the research effort going into the design of new greener and more sustainable chemical products will be insufficient to meet the demand that will result from legislation such as REACH. Our own efforts to engage the producers and retailers as well as the users of products containing chemicals in the green chemistry networks project, “Green Chemistry and the Consumer”, has made us aware of the very small amount of relevant research reported in the mainstream chemistry journals.5
The move from our well-established petrochemical based organics chemical industry to one based on renewable resources6,7 is beginning to open the door to numerous opportunities for exciting new chemistry research including benign extraction of valuable chemicals from biomass (e.g. using supercritical fluids),8 adding value to nature's most abundant polymers (starch, cellulose, chitin, etc) and the bulk conversion of biomass to new “bio-platform molecules” (Fig. 5). We recently reported methodology for physically expanding starch for example, to a very high surface area porous solid suitable for use in applications including as a catalyst support,9 as a stationary phase for chromatographic separation10 and in novel composite materials.11 Cellulose can also be expanded into a high surface area, mesoporous solid. Most recently we have found that controlled carbonisation of starch materials can lead to entirely new forms of carbon that we have nicknamed “starbons”12 (Fig. 6).
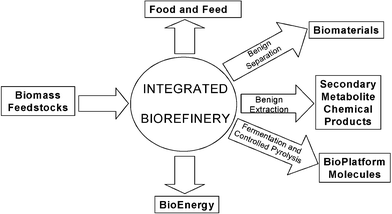 | ||
| Fig. 5 Green chemistry and the biorefinery. | ||
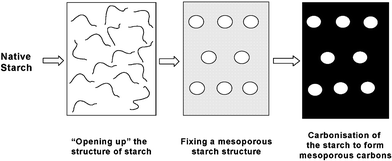 | ||
| Fig. 6 Schematic illustrations of the synthesis of mesoporous carbons “starbons”. | ||
It seems such a ‘waste’ that these diversely abundant and renewable materials currently have such low value uses. Nature's other large volume polymers cellulose13 and chitin14 also offer great potential for better exploitation.
Bioplatform molecules are the beginning of a new and vital challenge for organic chemists.15 Can we build on them as we have done over the last 70 years with the now well-established petro-platform molecules such as ethene and benzene (Fig. 7)? With the rapid growth in biotechnology we can expect to see both more candidates for new platform molecules and more selective bioprocesses for making platform molecules. We need a substantial growth in research activity on the conversion of these platform molecules to valuable products and including the effective use of the dilute ‘broths’ containing these molecules. The processing of these sustainable product mixtures needs careful control—we cannot afford to apply old, dirty, chemical methods and we must ensure that the overall environmental impact of the process from biomass to final product is kept low. The green processes involved are likely to be a mix of green chemical and biochemical (e.g. enzymatic) methods. I suggest that we need to carefully monitor this rapidly evolving technology for example through the use of “acceptability criteria”. These might, for example, state that there should be no more than three steps from the biomass to the final product and that each step should have a maximum E factor of 1 or 2 or similar green chemistry metric evaluation.
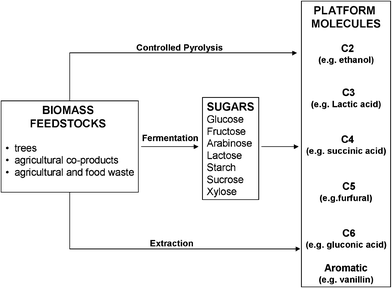 | ||
| Fig. 7 Bioplatform molecules. | ||
The green chemistry process research on novel catalysts, benign (non-VOC) solvents, etc is now being strengthened and moved closer to industrial application through the innovative contributions from chemical and process engineers, notably in the context of process intensification—scale out, not up (Fig. 8)!
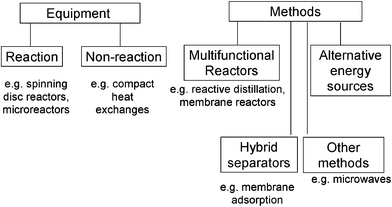 | ||
| Fig. 8 Process intensification. | ||
The growth in industrial utilisation of new, greener technologies in recent years is encouraging though it still only represents a tiny fraction of the total volume of chemical manufacturing worldwide. Interesting case studies are now available at all stages in the lifecycle (Fig. 9) and this journal regularly publishes good examples of research that should guarantee a pipeline of green chemistry processes that will make their way through to industrial applications. Highly publicised and sought after awards and prizes, notably the US Presidential Green Chemistry Awards play an important role in promoting good case studies and more should be encouraged especially with a product focus. Legislation is placing a new emphasis on chemical products that needs a strong response from industry and research. This should also include research grant programmes which focus on green product design and which encourage collaboration throughout the supply chain including a greater involvement from the user community. The importance and complexity of chemicals in cars and electronic equipment is now recognised in new legislation which affects the chemical inputs at manufacture (e.g. RoHS) and the fate of chemicals at end-of-life (e.g. end-of-life vehicle directives, WEEE). We should quickly extend this to other consumer goods—the thousands of different products in a modern department store represent an enormous contribution of chemicals to society and also an enormous challenge to meet new legislation and societal requirements to remove the more hazardous substances. This will force the search for substitutes for substances of concern, which in turn will reveal many gaps in the suitable chemicals that are available. How do we replace the more hazardous brominated flame retardants, surface primers, volatile organic solvents and plasticizers, which are so prevalent in the products of modern society? The challenges and opportunities ahead for green chemistry are greater than ever!
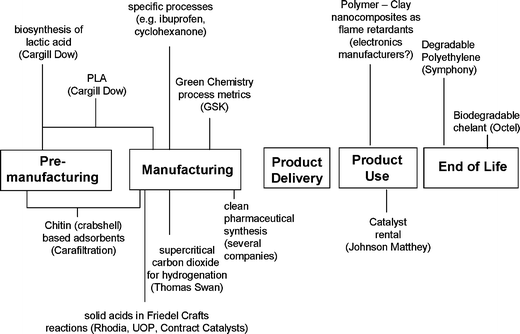 | ||
| Fig. 9 Examples of green chemistry in practice. | ||
The last 7 years have also seen a very welcome growth in the number of green chemistry centres, initiatives and networks across the world. I have been delighted to see major local activities start in countries and regions in Europe, Asia and the Americas. In many cases local activities have started with a very appropriate blend of education and research, reflecting the trend in the more established networks such as the GCN16 and the GCI.17 Education at all levels is vital to the future of green chemistry but while we see examples of good practice now in many countries and at school as well as tertiary level, the teaching of green chemistry at universities is still more the exception than the norm. We must ensure that the principles and practice of green chemistry are embedded in every chemical sciences degree course so that future generations of chemical scientists are at the heart of a sustainable 21st century society.
References
- J. H. Clark, Green Chem., 1999, 1, 1 RSC.
- http://europa.eu.int/comm./environment/chemical/reach.htm .
- http://www-european-voice.com/downloads/WithinREACHConferenceReport.pdf .
- Industrial Biotechnology and Sustainable Chemistry, Royal Belgian Academy Council of Applied Science, Brussels, January 2004 Search PubMed.
- Green Chemistry and the Consumer, http//www.york.ac.uk/inst.greenchemcic/gcc/GC&Chome.htm Search PubMed.
- Sustainable Development in Practice, ed. A. Azapagic, S. Podan and R. Clift, Wiley, Chichester, 2004 Search PubMed.
- Renewable Bioresources, ed. C. V. Stevens and R. G. Verte, Wiley, Chichester, 2004 Search PubMed.
- F. E. I. Deswarte, J. H. Clark, J. J. E. Hardy and P. M. Rose, Green Chem., 2006, 8 10.1039/b514978a.
- J. H. Clark, S. Doi, K. Milkowski and D. J. Macquarrie, Chem. Commun., 2002, 2632 RSC.
- V. Budarin, J. H. Clark, F. E. I. Deswarte, J. J. E. Hardy, A. J. Hunt and F. M. Kerton, Chem. Commun., 2005, 2869 RSC.
- K. Milkowski, J. H. Clark and S. Doi, Green Chem., 2004, 6, 189 RSC.
- UK Pat. Application, GB 0510471.6, 2005 Search PubMed.
- J. H. Clark, J. J. E. Hardy, K. Milkowski, F. M. Kerton, A. J. Hunt and F. E. I. Deswarte, PCT Int. Pat. Application, 2005 Search PubMed , WO 2005.011836.
- J. J. E. Hardy, S. Hubert, D. J. Macquarrie and A. J. Wilson, Green Chem., 2004, 6, 53 RSC.
- Top value added chemicals from biomass: http://www.eere.energy.gov/biomass/products_rd.html.
- http://www.chemsoc.org/gcn .
- http://www.chemistry.org/portal/a/c/s/1/acsdisplay.html?DOC= greenchemistryinstitute .
| This journal is © The Royal Society of Chemistry 2006 |
