Treatment of microbiologically polluted aquaculture waters by a novel photochemical technique of potentially low environmental impact
Michela
Magaraggia
*a,
Filippo
Faccenda
ab,
Andrea
Gandolfi
b and
Giulio
Jori
a
aDepartment of Biology, University of Padova, Via U. Bassi 58B, 35131, Padova, Italy. E-mail: michela.magaraggia@unipd.it
bIASMA Research Center, Limnology and Fish Research Unit, Natural Resources Department, Via Mach 1, 38010, San Michele a/Adige (TN), Italy
First published on 10th July 2006
Abstract
The applicability of a novel procedure for the disinfection of microbiologically polluted waters from fish-farming ponds, based on the combined action of visible light (including sunlight) and porphyrin-type photosensitising agents, has been investigated using (a) cell cultures of a Gram-positive bacterium (meticillin-resistant Staphylococcus aureus), a Gram-negative bacterium (Escherichia coli) and a fungal pathogen (Saprolegnia spp.); (b) pilot aquaculture plants involving either spontaneously or artificially Saprolegnia-infected rainbow trout (Oncorhynchus mykiss). The results obtained by using two cationic porphyrins, namely a tetra-substituted N-methyl-pyridyl-porphine (C1) and its analogue where one N-methyl group had been replaced by a N-tetradecyl chain (C14), and low intensity visible light irradiation showed an extensive (up to 6–7 log) decrease in the bacterial/fungal population after short incubation and irradiation times in the presence of micromolar photosensitiser concentrations. Moreover, C14 showed some toxic effect also in the absence of light. Extension of these studies to the pilot plants indicated that both C1 + light and C14 can prevent Saprolegnia infections or promote the cure of saprolegniasis in infected trout by treatments with submicromolar porphyrin doses. The procedure appears to be of low cost and to have a low environmental impact.
Introduction
It is well known that mycotic infections caused by members of Saprolegnia species, as well as by their close water mould relatives, represent a major challenge to a number of freshwater fishes and their eggs.1 Prevention and control of saprolegniasis are especially difficult, even under fish-farming conditions, owing to the ubiquitous nature and the rapid spreading of such fungi. The problem is further aggravated by various concomitant factors, such as (a) different stages of the fish life cycle are susceptible to saprolegniasis and in particular a marked increase in infection risk is observed during sexual maturation; (b) several forms of environmental stress, leading to a decrease in water quality (e.g. increased average temperatures, high organic loads), act as predisposing agents for the outbreak of the disease, often by weakening the innate defence systems of the fish; (c) many fungicides that are effective against higher fungi exhibit a low activity against oomycetes, (d) spores of the pathogen can successfully locate in the fish epidermis and, whilst the majority are removed or inactivated during quarantine in clear water, a significant number of viable fungal units persist on the body surface.2–4So far, mycotic infections arising in fisheries from Saprolegnia spp. have been most frequently treated by the application of malachite green, a triarylmethane dye that, either on its own or in combination with other biocides, was found to be the most effective out of many compounds tested against oomycete fungi.5,6 However, while still used in some countries due to its efficacy and low cost, recent concerns about the safety of this polycyclic aromatic derivative led to the banning of its use as a fisheries chemotherapeutant in many parts of the world.7 At present, formalin, a 24% aqueous solution of formaldehyde, represents the most frequently used disinfectant for prophylaxis of finned fish eggs and fry,8 and has been recently proposed as an effective treatment to reduce mortality in infected adult fish.9 The use of formalin, however, does not eliminate the risk for acute impact on ecosystems and potential harmful effects on human health,10 and is therefore discouraged, limited or even banned in several countries.
As a consequence, intensive investigations are being developed in order to find alternative strategies that allow a reasonably cheap and environmentally acceptable control of Saprolegnia infections in hatcheries.6,8,10,11–18
In this paper, we propose an innovative approach based on the combined action of two intrinsically non-toxic agents, namely visible light (or even sunlight) and porphyrin-type photosensitisers.19 Porphyrins are compounds of natural origin which, upon light-induced promotion from the ground to an electronically excited state, generate some hyper-reactive and highly cytotoxic oxygen species (mainly, singlet oxygen), which can attack a variety of cell constituents, including proteins, nucleotides, unsaturated lipids and steroids.20 The short lifetime of the photogenerated intermediates and the large number of possible targets restrict the range of the overall photoprocess to the close microenvironment of the photosensitiser.20,21
Recent studies resulted in the chemical synthesis of porphyrins or close analogues thereof, whose chemical structure was appropriately engineered in order to promote a fast and highly preferential binding with several types of microbial cells. Subsequent irradiation with light wavelengths specifically absorbed by the porphyrin causes an extensive mortality of a variety of pathogenic agents, such as Gram-positive and Gram-negative bacteria, fungi, mycoplasmas and parasites in either the cystic or the vegetative stage.22–24 Under suitable irradiation conditions, a 5–6 log decrease in the microbial population can be achieved with essentially no appreciable damage to the constituents of potential host tissues.25 Furthermore, porphyrins show no significant toxicity toward most higher organisms at photochemically active doses (namely, in the micromolar concentration range), as confirmed by their approved use as food additives26 or phototherapeutic agents for selected diseases in humans.27 Moreover, their excessive accumulation in the environment is unlikely owing to their gradual photobleaching by sunlight.28 Thus, it appeared of interest to investigate the efficacy and safety of this technique for the control of Saprolegnia infections in fish-farming pools. The results obtained in the present investigation appear to open novel promising perspectives for further developing such porphyrin-photosensitised processes into an efficacious technique for an environmentally friendly treatment of microbiologically polluted aquaculture systems.
Experimental
Porphyrins and other chemicals
Tetra-meso(N-methyl-pyridyl)porphine (C1) and its analogue Tri-meso(N-methyl-pyridyl), meso(N-tetradecyl-pyridyl)porphine (C14) were prepared by total chemical synthesis and kindly supplied from Frontier Scientific Inc. (Logan, Utah); both compounds were used without further purification, since HPLC analysis of the two porphyrin specimens according to standard literature procedures29 showed a purity higher than 98%. The chemical structure of the two porphyrins is shown in Fig. 1. The concentration of the porphyrin specimens was determined by measuring the absorbance at 424 nm in phosphate-buffered saline (PBS) using a molar extinction coefficient ε =194![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1. All other chemicals and solvents were commercially available reagents of at least analytical grade, unless stated otherwise.
000 M−1 cm−1. All other chemicals and solvents were commercially available reagents of at least analytical grade, unless stated otherwise.
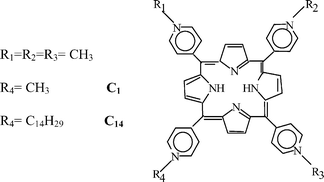 | ||
| Fig. 1 Chemical structure of the two meso-substituted porphyrins used as photosensitising agents. | ||
Bacterial and fungal cells
Staphylococcus aureus MRSA 110 and Escherichia coli 04 were grown aerobically at 37 °C in brain–heart infusion (BHI, Difco, Detroit, Michigan). The bacterial cells in the stationary phase of growth were harvested by centrifugation of broth cultures (2000 g for 15 min), washed twice with 10 mM PBS and diluted in the same buffer to an optical density of 0.7 at 650 nm, corresponding to 108–109 cells ml−1. Saprolegnia spp. strain, isolated from fish farming systems, was grown aerobically at 20 °C in glucose-yeast extract agar (GYA). For the various experiments, a small piece of agar was cut from the Petri dish and transferred into GY broth. After 48 h, the zoospores were harvested by centrifugation of broth cultures (2000 g for 15 min), washed twice with PBS and diluted with the same buffer to an optical density of 0.7 at 530 nm corresponding to about 2 × 108 zoospores ml−1.Fish
Adult rainbow trout, Oncorhynchus mykiss, from a commercial stock were transferred and acclimatised at the IASMA Fish Research facility. Fish were maintained in a single 1000 l tank at 13 °C and daily fed about 1.0% of their total body weight until the day before the experiment started. Both artificially and spontaneously infected trout were studied. In all cases, the fishes were treated in compliance with the rules for humane treatment of experimental animals established by the ethical committee of the University of Padova. To obtain the artificial infection, fish were lightly anaesthetised by exposure to a ternary mixture of 0.2 g l−1 2,2,2-trichloro-butanol and 0.2 g l−1 of 50 ∶ 50 (v/v) acetone-chloroform (Scubla Aquaculture, Remanzacco, Italy). The skin infection on the trout epidermis was obtained by scraping scales from about 2 cm2 of the dorsal region, between the adipose and the caudal fin, and by direct contact of the lesion with mycelium wads. Presence or absence of the infection was evaluated by the occurrence of cottony-white patches on the lesion of the fish after one and two weeks from the artificial infection procedure.Only sixteen naturally infected individuals were available for the spontaneous infection trial. These trout showed a cottony-white mycelium infection at the beginning of the experiment, and regression of the infection was evaluated after the various treatments.
Porphyrin uptake studies
Samples containing 108–109 cells or zoospores ml−1 were prepared by dilution of a bacterial or mycelium suspension previously washed in PBS at pH 7.2. Each sample (5 ml of cell suspension) was incubated with different porphyrin concentrations for 30 min in the dark at room temperature. After incubation, 50 μl of each cell suspension were serially 10-fold diluted in PBS and the number of colonies found after 18–24 h incubation at 37 °C were counted. The remaining suspensions were centrifuged and washed twice with PBS by centrifugation. The amount of cell-bound porphyrins was determined in the pellets after resuspension and overnight-incubation in 2% aqueous sodium dodecylsulfate (SDS). The porphyrin concentration in the cell lysate was determined by measuring the intensity of the porphyrin-typical red fluorescence (600–750 nm) emitted after excitation at 420 nm and interpolating the data on a calibration plot obtained with known porphyrin concentrations. The amount of porphyrin bound to the cells was expressed as nmoles of porphyrin per 108 cells. Control studies showed that this procedure allows the recovery of at least 95% of the cell-bound porphyrin.Irradiation experiments and survival studies with microbial cells and zoospores
Suspensions (50 μl) of S. aureus MRSA, E. coli 04 and Saprolegnia spp. containing approximately 2 × 108 colony forming units (CFU) ml−1 in PBS were transferred into wells of a microtitre plate. The same volume of a porphyrin solution in PBS was added to each well to a final porphyrin concentration ranging from 0.05 to 10 μM. Samples were incubated at room temperature in the dark for 0.5–1 h, then irradiated by means of white light (400–800 nm) isolated from the emission of a quartz-halogen lamp equipped with UV and infrared filters (Teclas, Lugano, Switzerland). The irradiations were performed for 20 min at a fluence-rate of 100 mW cm−2, as measured with a radiometer (IL 1700, International Light, Newburyport, Massachusetts), which was calibrated for measurements in the 320–750 nm wavelength range. At the end of the irradiation experiments, unirradiated (controls) and irradiated bacterial cells or zoospores were serially 10-fold diluted in PBS and the number of bacterial and fungine colonies found after 18–24 h incubation at 37 and 20 °C, respectively, were counted.Photostability studies
The photostability of the porphyrins was determined in PBS upon illumination of a 2 μM solution (initial absorbance around 0.4 at 424 nm) with the Teclas lamp. The light source was operated at a fluence-rate of 100 mW cm−2. During irradiation the solution was magnetically stirred at room temperature. The concentration of the porphyrin samples was monitored spectrophotometrically at different irradiation times, and the photostability was expressed as the percent residual absorbance referred to the absorbance measured before irradiation.In vivo experiments on fish
The treatment of fish with porphyrins and visible light irradiation in pilot plants was performed with a two-fold aim: (a) to prevent the onset of fish infections; and (b) to treat infections developed by fish that had been naturally exposed to Saprolegnia.In the first group the individuals were not scraped and were maintained as a general control (uninfected control) of the health status of the stock. In the second group the individuals received no other treatment than artificial infection (infected control). In the third group the artificially infected individuals were dark incubated for 10 min with 0.44 μM (0.6 mg l−1) C1 doses. The tank was then irradiated for 1 h, with two 500 W quartz-halogen lamps emitting white light (400–800 nm) which were operated at a constant fluence-rate of 50 mW cm−2 as measured by means of a radiometer placed at the level of the water surface. During the whole incubation/irradiation period, the water (total volume 1000 l) was kept in a closed circuit and recirculated by a motor-driven pump, and its temperature was monitored and maintained at 13 °C. At the end, the normal flow of circulating water was restarted. The incubation/irradiation treatment was repeated at daily intervals for ten consecutive days, starting from the first day after the infection.
In the fourth group the artificially infected individuals were incubated for 10 min with 0.2 μM (0.3 mg l−1) C14 using the above described closed circuit, after which the standard water flow was restored. The procedure was repeated for ten consecutive days, however no irradiation was used.
The first group was maintained as an untreated control in a 1000 l tank.
The second group was dark incubated with 0.6 mg l−1 C1 for 10 min in an 80 l pool and irradiated for 1 h using the same water-recirculating procedure as described above. The irradiation was performed by using the 400–800 nm wavelength interval emitted from two 100 W incandescent filament lamps and the water temperature was kept at 13 °C throughout the light exposure. The treatment was daily repeated for six consecutive days. At predetermined intervals, water samples were taken for analysis of the microbial charge. After each treatment repetition, fish were moved to a 1000 l tank. The third group was incubated with 0.6 mg l−1 C14 for 24 h in a 150 l tank and not irradiated. After this single treatment, fish were moved to a 1000 l tank.
Given the exiguous number of available naturally infected individuals no statistical evaluation of results was possible but, even if preliminary, the experiment represented a still informative first test of the curative approach.
Results
Physico-chemical studies
The absorption spectrum of C1 porphyrin in the visible wavelength range is shown in Fig. 2a. As it is typical of meso-substituted porphyrin derivatives,30 the spectrum is characterized by an intense absorption band in the blue wavelength interval (the so called Soret band, max. around 420 nm), as well as by a sequence of less pronounced bands in the green and red spectral region. The absorption spectrum of C14 was essentially superimposable with that of the C1 porphyrin. Therefore, porphyrins appear to be particularly efficient absorbers of a very broad fraction of sunlight or artificially produced visible light. Both porphyrin samples also display a fluorescence emission in the red region (Fig. 2b), which can be useful for detecting the presence and measuring the concentration of these compounds in biological systems as well as in water specimens, owing to the high sensitivity of this spectroscopic technique. | ||
| Fig. 2 (a) Visible absorption spectrum of 1 μM C1 porphyrin in phosphate-buffered saline (PBS). (b) Fluorescence emission spectrum of 1 μM C1 in PBS, excitation at 420 nm (a.u. = arbitrary units). | ||
Porphyrin uptake studies
The effect of C1 and C14 concentration on the uptake of the porphyrins by Saprolegnia zoospores after 30 min incubation is shown in Fig. 3a,b. The amount of cell-bound C1 steadily increases with the photosensitiser concentration in the incubation medium, up to at least 5 μM. The efficiency of the uptake process is strongly influenced by the length of the N-alkyl chain, as one can see in Fig. 3b. C14 is accumulated by Saprolegnia zoospores in significantly larger amounts as compared with C1 and no saturation of porphyrin binding to the cells is observed at least up to a 10 μM concentration in the incubation medium. Similar observations were made upon incubation of C1 and C14 with either MRSA or E. coli cells, as shown in Table 1. | ||
| Fig. 3 Effect of the porphyrin concentration on the uptake of C1 (a) and C14 (b) by Saprolegnia spp. cells after a 30 min incubation in the dark. | ||
| Bound porphyrin/nmoles (108 cells)−1 | ||
|---|---|---|
| Porphyrin | MRSA | E. coli |
| C1 | 0.03 ± 0.01 | 0.04 ± 0.00 |
| C14 | 0.32 ± 0.05 | 0.17 ± 0.03 |
Irradiation experiments and survival studies with microbial cells and zoospores
C1 exhibited a relatively low phototoxicity towards Saprolegnia: the cell survival decreased by only two logs after 20 min irradiation in the presence of a 10 μM porphyrin concentration (Fig. 4a). On the other hand, C14 appeared to be endowed with a significantly larger photocytotoxic action: in particular, the survival of the fungal cells decreased by about 6 logs after phototreatment with 10 μM C14 (Fig. 4b). While the C1-incubated and unirradiated controls showed no decrease in survival at all the porphyrin concentrations tested by us, the C14 appeared to induce some cytotoxic effects at concentrations around 10 μM even in the absence of visible light-irradiation (e.g. 1 log decrease in survival after 30 min incubation). The same trend was found upon photosensitisation of MRSA or E. coli: as one can see in Table 2, C1 exhibited a higher phototoxicity towards these bacterial cells than towards Saprolegnia, even though C14 still displayed a more powerful overall photosensitising activity. Moreover, as pointed out by previous studies,19 incubation of MRSA or E. coli for 30 min with 8 μM C14 in the dark caused an about 2 log drop in cell viability.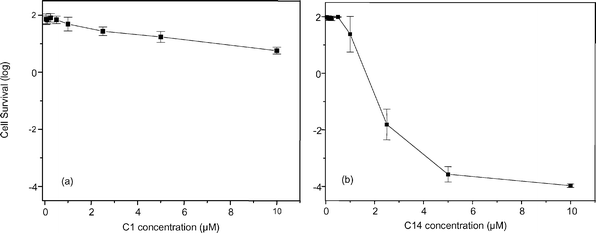 | ||
| Fig. 4 Effect of C1 (a) and C14 (b) concentration on the survival of Saprolegnia spp. cells after 30 min dark incubation and 20 min irradiation with white light (Teclas lamp) at a fluence rate of 100 mW cm−2. | ||
| Decrease of survival (log) | ||
|---|---|---|
| Porphyrin | MRSA | E. coli |
| C1 1 μM | −3.40 ± 0.18 | 0.00 ± 0.00 |
| 10 μM | −6.82 ± 0.31 | −5.18 ± 0.40 |
| C14 1 μM | −6.76 ± 0.33 | −3.63 ± 0.25 |
| 10 μM | −7.00 ± 0.00 | −7.00 ± 0.00 |
Photostability studies
As shown in Fig. 5a,b the two porphyrins underwent a gradual photobleaching upon exposure to full spectrum white light in a PBS-based medium. The absorbance decrease involved all the envelope of visible bands of C1 and C14, indicating that the photoprocess induces a destruction of the aromatic macrocycle with no formation of new visible light-absorbing products. C1 exhibited a slightly smaller degree of photosensitivity than C14, since a 6% photodegradation occurred after 5 min irradiation against a 12% photodegradation of C14. Solutions of C1 and C14 which had been extensively (>80%) photobleached were incubated in the dark with human HT-1080 fibroblasts with no measurable decrease in cell survival, which suggests that the photodegradation products are devoid of any significant toxicity. | ||
| Fig. 5 Effect of the irradiation time on the absorption properties of 2 μM C1 (a) and C14 (b) solutions in PBS which were exposed to white light (Teclas lamp) at a fluence rate of 100 mW cm−2. | ||
In vivo experiment on fish
Mycotic infections are often developed in fish that present epidermal lesions. In actual fact, about 27% (8/30) of inoculated control fish, where lesions had been induced in the dorsal region by scraping, underwent the expected infection by Saprolegnia, which was detected by the formation of a cottony-white mycelial mass after one week (Fig. 6a, a more detailed view of the infection is shown in Fig. 6a′). The experimental treatment with C1 and light (Fig. 6b and b′) or C14 without irradiation (Fig. 6c and c′) following the preventive protocol as described in the Experimental determined a reduction of the infected percentage to 10% and 13%, respectively, after one week. Even though the percentage of infection was low in our system, these results indicate (a) the feasibility of our experimental approach, and (b) a positive trend as regards the effect of C1 and C14 on the control of saprolegniasis. Additional more in-depth experiments are in preparation. It is to be underlined that the results were obtained by using C1 or C14 concentrations which were significantly lower than those giving optimal antimicrobial effects in cell culture experiments: this choice was due to several factors, such as the smaller population of pathogens in the pilot plant systems, the need to ensure a sufficient illumination of deeper water layers which would be precluded by excessive absorbance of the porphyrins, and the opportunity to minimise the risks of direct damage of the porphyrins to the fishes. After two weeks, all the infected individuals from all the experimental groups recovered from the infection.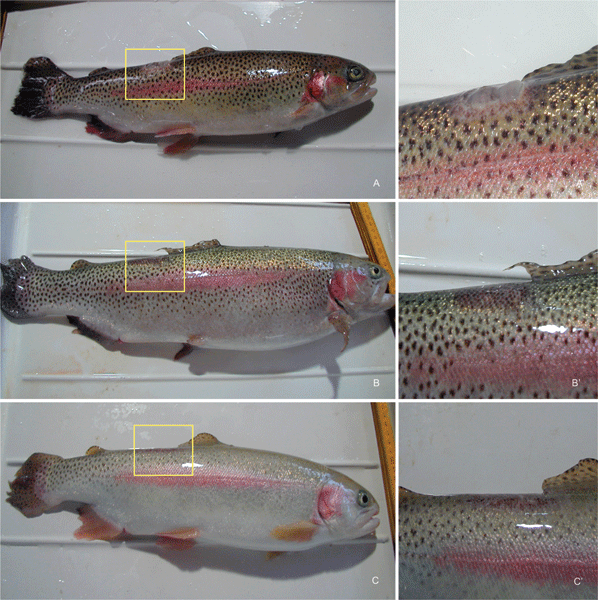 | ||
| Fig. 6 (a) Example of a trout which was infected by inoculation of Saprolegnia into an artificially induced lesion in the dorsal region. The infection appears as a cotton-type mycelial mass as shown in the magnified picture, 6a′. (b) Example of a Saprolegnia-infected trout that has been treated by 0.6 mg l−1 C1 + light according to the preventive protocol. (c) Example of a Saprolegnia-infected trout that has been treated with 0.3 mg l−1 C14 according to the preventive protocol. The magnified images 6b′ and 6c′ show no detectable appearance of fungal infection. | ||
On the other hand, when trout that had developed a spontaneous infection by Saprolegnia (Fig. 7a), were added with C1 and exposed to visible light or added with C14 according to the curative protocol, a complete remission of the infection was induced within one week. This was followed by the complete healing of the ulcerated lesion, which had been formed after elimination of the mycelial mass (Fig. 7b). Analysis of the water samples obtained from such plants before and after the treatment showed that the treatment with C1 and light and C14 induced an about 2 log decrease in the population of the overall microbial population. In no case, recurrence of the saprolegniasis took place in the previously infected or other sites of the fish. On the other hand, no spontaneous remission of saprolegniasis was observed in the infected but untreated control fish.
 | ||
| Fig. 7 (a) Example of Saprolegnia infection spontaneously developed in farmed trout. (b) The same infected trout after 1 week treatment with 0.6 mg l−1 C1 + white light according to the curative protocol: the picture shows the total disappearance of the mycelium, which was then followed by a complete healing of the initially formed ulceration. | ||
Discussion
The results obtained in the present investigation clearly suggest that the combination of cationic porphyrins and visible light could represent a viable and environmentally friendly alternative for the control of the population of potential pathogens in waters from fish farming ponds. The main favourable features of this technique can be summarised as follows.(1) The photosensitizing and cytocidal activity of both C1 and C14 appears to be characterized by a broad spectrum, since an extensive drop in the population of a variety of microorganisms has been achieved by irradiation of selected representatives of Gram-positive or Gram-negative bacteria, including an aggressive and antibiotic-resistant bacterium such as MRSA, as well as of a well known fungal pathogen such as Saprolegnia spp. As mentioned in the Introduction, such a behaviour is typical of this class of compounds. Quite interestingly, the C14 derivative exhibits an appreciable cytotoxic action even in the dark; this effect has been ascribed to the presence of the long hydrocarbon tail which can interact with hydrophobic areas in the cell membrane, thereby inducing a marked alteration of the native three-dimensional architecture and impairing specific metabolic processes.31 Of course, the antimicrobial activity of C14 is further enhanced by visible light irradiation (Fig. 4), hence this compound appears to be especially promising given its large affinity for both bacteria and fungi (Fig. 3 and Table 1) and its high antimicrobial efficiency, which should allow the use of particularly low dosages.
(2) The multi-target nature of the mode of action typical of porphyrins (see the Introduction) makes it very unlikely that photoresistant strains of bacterial and fungal cells are selected.23,32 Thus, up to ten consecutive generations of bacteria have been exposed to the photosensitising action of cationic porphyrins with no detectable modification of their degree of photosensitivity.33
(3) At the same time, the presence of the flat aromatic macrocycle favours the partitioning of porphyrin derivatives in the lipid domains of cell membranes or their stable association with a variety of proteins;23,34 as a consequence, exogenously administered porphyrins have a high probability to be “intercepted” before significant concentrations reach the genetic material. All the evidence available so far indicates that photoprocesses promoted by porphyrins have a very low mutagenic potential. This feature is important for the safety of the procedure in the case of repetitive treatments.
(4) The data obtained with cell cultures were preliminarily confirmed by the experiments carried out in a pilot aquaculture plant. The addition of C1 followed by visible light irradiation or C14 in the dark prevents the onset of saprolegniasis in at least 50% of the trout that would otherwise be infected by this parasite under our experimental conditions (Fig. 6); clearly, the porphyrins were able to interact with the pathogen cells that had colonized the lesion and either cause their inactivation or prevent their proliferation. Moreover, the C1 + visible light and the C14 treatments induced the disappearance of the disease in trout that had been heavily infected by Saprolegnia spp. (Fig. 7). It appears reasonable to hypothesize that this effect reflects the toxic action of the added porphyrins on pathogens possibly present in the system, as confirmed by the observed higher than 90% decrease in the microbial population in the water of the trout-farming pond. This result is achieved by using mild experimental conditions and is not accompanied by important photoinduced damage at the level of the perilesional tissues, as shown by the ready and complete healing process. Remarkably, either an incubation time of 1 h per day or a single 24 h incubation are sufficient to generate satisfactory levels of either preventive or curative effects. Further experiments, with increased number of naturally infected fish or with higher percentages of artificially infected fish, are necessary. To this aim, temperature increase has recently been successfully used as an effective stressor to induce saprolegniasis in fish.9
(5) The porphyrins studied by us display no significant toxicity toward adult trout at photochemically active doses (C1) or at doses which induce a marked mortality of microbial pathogens (C14). This finding is in agreement with previous observations dealing with the effect of porphyrins on a variety of higher organisms,35 which justifies their increasing utilisation as food additives or phototherapeutic agents. Several porphyrins and their chlorin analogues are widespread in numerous ecosystems and their toxicity to cells and tissues becomes important only at millimolar concentrations,36 namely for dosages which are 2–3 orders of magnitude larger than those yielding a satisfactory antimicrobial effect both on cell cultures and in pilot plants.
(6) The intense absorption band of porphyrins in the 420–430 nm spectral region (Fig. 2a) allows a very efficient interaction with blue light wavelengths, which are endowed with maximum penetration power into natural waters out of the wavelengths which are present in the solar spectrum.37 On the basis of the Beer–Lambert law, one can estimate that for a porphyrin concentration of 0.44 μM, as used by us, and an ε value of 194![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1cm−1 the absorption A of the incident light will be around 0.085 mol−1 dm3 cm−1; since total light absorption by a system corresponds with A
= 2.0–2.2 mol−1 dm3 cm−1, the illumination can be assumed to be sufficiently uniform to a water depth of about 25 cm. Less efficiently absorbed wavelengths (e.g. those in the green and red spectral regions) will allow the illumination of even larger water volumes, even though the smaller probability of porphyrin photoexcitation by such wavelengths may require longer light exposure times. Moreover, in the case of particularly deep or scarcely transparent fish farming pools, the control of the pathogen population can be reinforced by addition of C14 in suitable doses, since the latter porphyrin appears to induce cytotoxic effects even in the dark.
000 M−1cm−1 the absorption A of the incident light will be around 0.085 mol−1 dm3 cm−1; since total light absorption by a system corresponds with A
= 2.0–2.2 mol−1 dm3 cm−1, the illumination can be assumed to be sufficiently uniform to a water depth of about 25 cm. Less efficiently absorbed wavelengths (e.g. those in the green and red spectral regions) will allow the illumination of even larger water volumes, even though the smaller probability of porphyrin photoexcitation by such wavelengths may require longer light exposure times. Moreover, in the case of particularly deep or scarcely transparent fish farming pools, the control of the pathogen population can be reinforced by addition of C14 in suitable doses, since the latter porphyrin appears to induce cytotoxic effects even in the dark.
(7) The overall treatment time is further reduced by the short incubation which is required in order to achieve a significantly large association of the porphyrin photosensitiser with the microbial cells. Such a fast kinetic process is permitted by the fact that the binding is of electrostatic nature, namely it is promoted by the real time interaction between the positively charged peripheral substituents in the porphyrin molecule and the large number of negatively charged functional groups (e.g. lipopolysaccharides, lipoteichoic and teichuronic acids) which are present in the outer wall of many microbial cells. Thus, previous investigations showed that prolonging the incubation of porphyrin-type derivatives with microbial cells from a few minutes to 1–2 h brings about no measurable increase in the amount of cell-bound antimicrobial agent.38 The close proximity between the porphyrin and the potential targets of the photosensitised process represents an important factor for enhancing the efficacy of the treatment, since several photosensitive sites will be within the normal diffusion range (<0.1 μm)39 of the photogenerated reactive oxygen species, such as singlet oxygen.
(8) The water disinfection can be successfully obtained by using a simple and inexpensive technology. In actual fact, an extensive decrease in microbial population is achieved by using low light intensities, of the order of 50 mW cm−2, which can be easily reached by irradiation with halogen or incandescent filament lamps, that is light sources of low cost, which have a long life span (a few thousand hours) and require no protective measures for the operators, the fishes and the consumers. Moreover, the low fluence-rate causes no detectable thermal effect, thus avoiding any problems related with the possible increase in water temperature. In our pilot plant studies, the temperature of the water was controlled at a constant level of 13 °C simply to guarantee optimal living conditions for the trout. At the same time the high efficiency of light absorption and microbial cell photosensitization by porphyrins allows the use of low dosages: the meso-substituted derivatives used in the present investigation are presently produced in relatively small amounts for medical use; it is to be expected that their cost will significantly drop once they are produced on the very large scale required for water treatment.
(9) Lastly, the proposed methodology appears to be environmentally friendly, since it is based on the use of sunlight or sunlight simulators in combination with antimicrobial agents of natural origin. In any case, the accumulation of porphyrins in the various ecosystems is unlikely owing to their gradual photobleaching under the action of ambient light, as shown by our present studies and as repeatedly reported in the literature.19 Moreover, the ready water solubility of C1 and C14 should guarantee their fast dilution in the ground to levels which are below any risk threshold. In any case, the photobleaching products of these porphyrins also show no major toxic effects at least against mammalian cells. Typically, meso-substituted porphyrins do not undergo biodegradation since steric factors prevent the interaction with the active site of enzymes involved in porphyrin catabolism, again ruling out the possibility of widespread toxicity induced by accumulation of such products in the environments.40
Conclusions
Through studies with cell cultures of potential pathogenic agents for fish-farming systems (including both bacteria and fungi), as well as with pilot aquaculture plants containing either artificially or spontaneously Saprolegnia-infected trout, a method has been developed which is suitable for preventing the onset or propagation of the disease and/or to induce its regression. The method relies on the combined action of two intrinsically non-toxic agents, such as porphyrin photosensitisers and visible light, and is active on both wild and antibiotic-resistant strains; in particular, the proposed methodology appears to have no major effects on the fishes and to be endowed with several favourable features to minimize its impact on the environment. The approach should be also considered in connection with findings by previous investigators, showing that visible light-excited cationic porphyrins can efficiently induce the destruction of helminth eggs41 and fecal bacteria42 in waste waters. Thus, porphyrin photosensitisation appears to represent a very useful and flexible tool for the decontamination of microbiologically polluted waters. The observed improvement in the overall performance when C14 was used in the place of C1 warrants a thorough study on the relationships between chemical structure of the porphyrins and their efficiency in the dark- or photo-disinfection of water, also in view of the possible development of protocols tailored to a given type of water or pathogen contaminant.Acknowledgements
Ivan Stocchetti, Walter Delvai and Nicola Merlo are kindly acknowledged for their technical support during experiments on fish. This work was supported in part by a NATO grant EST CLG 981136. The discussions and suggestions provided by Prof. T. Ben Amor are gratefully acknowledged.References
- D. J. Alderman, in Salmon Saprolegniasis, ed. G. J. Mueller, USDOE Bonneville Power Administration, Portland, OR, 1994, pp. 111–129 Search PubMed.
- L. G. Willoughby and R. J. Roberts, J. Fish Dis., 1992, 15, 1–13 Search PubMed.
- K. Hatai, L. G. Willoughby and G. W. Beakes, Mycol. Res., 1990, 94, 182–190 CrossRef.
- K. Hatai, in Proceedings of the OJI International Symposium on Salmonid Diseases, ed. T. Kimura, Hokkaido University Press, Sapporo, 1992, pp. 283–299 Search PubMed.
- D. J. Alderman, in Aquaculture and Environment, European Aquaculture Society Special Publication 16, ed. N. De Pauw and J. Joyce, EAS, Bruxelles, 1992, pp. 235–244 Search PubMed.
- R. E. Campbell, J. H. Lilley, V. Panyawachira and S. Kanchanakhan, Aquacult. Res., 2001, 32, 223–233 Search PubMed.
- S. Srivastava, R. Sinha and D. Roy, Aquat. Toxicol., 2004, 66, 319–329 CrossRef CAS.
- G. Forneris, S. Bellardi, G. B. Palmegiano, M. Saroglia, B. Sicuro, L. Gasco and I. Zoccarato, Aquaculture, 2003, 221, 157–166 CrossRef CAS.
- C. M. Gieseker, S. G. Serfling and R. Reimschuessel, Aquaculture, 2006, 253, 120–129 CrossRef CAS.
- T. M. Schreier, J. J. Rach and G. E. Howe, Aquaculture, 1996, 140, 323–331 CrossRef CAS.
- L. L. Marking, J. J. Rach and T. M. Schreier, Prog. Fish-Cult., 1994, 56, 225–231 Search PubMed.
- M. M. A. Hussein, S. Wada, K. Hatai and A. Yamamoto, J. Aquat. Anim. Health, 2000, 12, 224–229 Search PubMed.
- J. A. Cooper, J. M. Pillinger and I. Ridge, Aquaculture, 1997, 156, 157–163 CrossRef.
- M. Miura, H. Oono, N. Tuchida, K. Hatai and T. Kiryu, Fish Pathol., 2005, 40(2), 81–86 Search PubMed.
- E. Branson, Vet. Rec., 2002, 151, 539–541 CAS.
- T. G. Pottinger and J. G. Day, Dis. Aquat. Org., 1999, 36, 129–141 Search PubMed.
- S. Perrucci, S. Cecchini, C. Pretti, A. M. Varriale Cognetti, G. Macchioni, G. Flamini and L. Cioni, Phytother. Res., 1995, 9, 147–149 CrossRef CAS.
- M. P. Tampieri, R. Galuppi, M. Caffara and J. Malvisti, Boll. Soc. It. Patol. Ittica, 1999, 26, 10–17 Search PubMed.
- G. Jori and J. D. Spikes, in Topics in Photomedicine, ed. K. C. Smith, Plenum Press, New York, 1984, pp. 183–318 Search PubMed.
- R. Bonnett, Chem. Soc. Rev., 1995, 24, 19–33 RSC.
- C. S. Foote, Photochem. Photobiol., 1998, 54, 659–660.
- G. Jori and S. B. Brown, Photochem. Photobiol. Sci., 2004, 3, 403–405 RSC.
- T. Maisch, R. M. Szeimies, G. Jori and C. Abels, Photochem. Photobiol. Sci., 2004, 3, 907–917 RSC.
- K. Kassab, D. Dei, G. Roncucci, G. Jori and O. Coppellotti, Photochem. Photobiol. Sci., 2003, 2, 668–672 RSC.
- M. Soncin, C. Fabris, A. Busetti, D. Dei, D. Nistri, G. Roncucci and G. Jori, Photochem. Photobiol. Sci., 2002, 1, 815–819 RSC.
- Certified Colour Industry Committee, Food Technol., 1988, 22, 15.
- T. Patrice, Photodynamic Therapy, Royal Society of Chemistry, Cambridge, 2003 Search PubMed.
- R. Rotomskis, G. Streckyte and S. Bagdonas, J. Photochem. Photobiol., B, 1997, 39, 172–175 CrossRef CAS.
- J. H. Fuhrhop and K. M. Smith, in Porphyrins and Metalloporphyrins, ed. K. M. Smith, Elsevier, Amsterdam, 1975, pp. 757–869 Search PubMed.
- E. Reddi and G. Jori, Rev. Chem. Intermed., 1988, 10, 241–268 Search PubMed.
- E. Reddi, M. Ceccon, G. Jori, J. C. Bommer, F. Elisei, L. Latterini and U. Mazzucato, Photochem. Photobiol., 2002, 75, 462–470 CrossRef CAS.
- Z. Malik, J. Hanania and Y. Nitzan, J. Photochem. Photobiol., B, 1990, 5, 281–293 CrossRef CAS.
- F. M. Lauro, P. Pretto, L. Covolo, G. Jori and G. Bertoloni, Photochem. Photobiol. Sci., 2002, 1, 468–470 RSC.
- M. Wainwright, J. Antimicrob. Chemother., 1998, 42, 13–28 CrossRef CAS.
- N. Solban, B. Ortel, B. Pogue and T. Hasan, Ernst Schering Res. Found. Workshop, 2005, 49, 229–258 Search PubMed.
- J. G. Moser, in Photodynamic Tumour Therapy, ed. J. G. Moser, Harwood Academic Publishers, London, 1997, pp. 3–8 Search PubMed.
- K. S. Baker and R. C. Smith, in The Role of Solar Radiation in Marine Ecosystems, ed. J. Calkins, Plenum Press, New York, 1982, pp. 233–246 Search PubMed.
- M. Salomon-Divon, Y. Nitzan and Z. Malik, Photochem. Photobiol. Sci., 2004, 3, 423–429 RSC.
- J. Moan and K. Berg, Photochem. Photobiol., 1991, 53, 549–553 CrossRef CAS.
- A. R. Battersby and E. McDonald, in Porphyrins and Metalloporphyrins, ed. K. M. Smith, Elsevier Co., Amsterdam, 1985, pp. 61–122 Search PubMed.
- Z. Alouini and M. Jemli, J. Environ. Monit., 2001, 3, 548–551 RSC.
- M. Jemli, Z. Alouini, S. Sabbahi and M. Gueddari, J. Environ. Monit., 2002, 4, 511–516 RSC.
| This journal is © The Royal Society of Chemistry 2006 |
