Contamination of Canadian and European bottled waters with antimony from PET containers
William
Shotyk
*,
Michael
Krachler
and
Bin
Chen
Institute of Environmental Geochemistry, University of Heidelberg, INF 236, D-69120, Heidelberg, Germany. E-mail: shotyk@ugc.uni-heidelberg.de; Fax: 54 5228; Tel: +49 (6221) 54 4803
First published on 20th January 2006
Abstract
Using clean lab methods and protocols developed for measuring Sb in polar snow and ice, we report the abundance of Sb in fifteen brands of bottled water from Canada and forty-eight from Europe. Comparison with the natural abundance of Sb in pristine groundwaters, water bottled commercially in polypropylene, analyses of source waters prior to bottling, and addition of uncontaminated groundwater to PET bottles, provides unambiguous evidence of Sb leaching from the containers. In contrast to the pristine groundwater in Ontario, Canada containing 2.2 ± 1.2 ng l−1 Sb, 12 brands of bottled natural waters from Canada contained 156 ± 86 ng l−1 and 3 brands of deionized water contained 162 ± 30 ng l−1; all of these were bottled in PET containers. Natural water from Ontario bottled in polypropylene contained only 8.2 ± 0.9 ng l−1. Comparison of three German brands of water available in both glass bottles and PET containers showed that waters bottled in PET contained up to 30 times more Sb. To confirm that the elevated Sb concentrations are due to leaching from the PET containers, water was collected in acid-cleaned LDPE bottles from a commercial source in Germany, prior to bottling; this water was found to contain 3.8 ± 0.9 ng l−1 Sb (n = 5), compared with the same brand of water purchased locally in PET bottles containing 359 ± 54 ng l−1 (n = 6). This same brand of water in PET bottles, after an additional three months of storage at room temperature, yielded 626 ± 15 ng l−1 Sb (n = 3). Other German brands of water in PET bottles contained 253–546 ng l−1 Sb (n = 5). The median concentration of Sb in thirty-five brands of water bottled in PET from eleven other European countries was 343 ng l−1 (n = 35). As an independent check of the hypothesis that Sb is leaching from PET, the pristine groundwater from Canada (containing 2.2 ± 1.2 ng l−1 Sb) was collected from the source using PET bottles from Germany: this water contained 50 ± 17 ng l−1 Sb (n = 2) after only 37 days, even though it was stored in the refrigerator, and 566 ng l−1 after six months storage at room temperature.
Introduction
Antimony is a potentially toxic trace element with no known physiological function, but its natural and anthropogenic geochemical cycles are poorly understood.1 Found at the surface of the earth mainly in the form of relatively insoluble metal sulfides, its abundance in crustal rocks (ca. 0.3 mg kg−1) is lower than that of Pb (15 mg kg−1) or As (1.5 mg kg−1), two elements with which Sb is often compared.2 We have recently shown that the natural abundance of Sb in uncontaminated groundwaters may be very low. For example, in pristine groundwaters from a calcareous aquifer in southern Ontario, Canada,3 the average Sb concentration was only 2.2 ± 1.2 ng l−1 (n = 34). The reliable measurement of Sb at these concentrations was only made possible by the recent development of procedures and methods for measuring Sb in polar snow and ice,4 including the use of clean lab facilities, metal-free Class 100 laminar flow clean air cabinets, and inductively coupled plasma-sector field mass spectrometry (ICP-SMS). In this regard, the limit of detection (LOD) which has been achieved (0.03 ng l−1) allows Sb to be measured in even the most dilute geological and biological fluids.In contrast to the very low concentrations of Sb recently presented for pristine groundwaters, most published studies of Sb in bottled waters report much higher values.3 For example, in a study of bottled waters from Canada, Dabeka et al.5 found that 42 mineral waters averaged 320 ng l−1 Sb and 102 springwaters averaged 300 ng l−1; these average values are more than 100 times greater than average abundance of Sb which we found3 in pristine groundwaters from southern Ontario, Canada (2.2 ± 1.2 ng l−1). In a study of 56 bottled waters from Europe, the median Sb concentration was 165 ng l−16 which is high compared to its abundance in groundwaters from Norway where values are typically on the order of ca. 30 ng l−1 but often less than 2 ng l−1.7 A study of Sb in bottled waters from Japan reported Sb above the limit of detection (500 ng l−1) in 16 out of 55 brands.8 Comparison of our data for pristine groundwaters from Canada (2.2 ± 1.2 ng l−1) with published data for bottled waters leads us to ask whether the Sb concentrations reported for the bottled waters truly reflect the Sb concentrations originally present in the natural waters prior to bottling, or whether they possibly reflect an additional contribution from the containers in which many of the waters are sold.
The reasons for the large concentration differences between pristine groundwaters and bottled waters warrants critical examination. While geological differences certainly may play a role, we suspect that part of the difference is because of the inadequate LOD provided by most of the analytical methods employed in the past for measuring Sb in waters, including GFAAS, HG-AAS, HG-AFS, ICP-QMS, and even INAA.3 However, a complicating factor is the widespread use of Sb in the manufacture of plastics, and the effects of these on laboratory blank values appear to have been inadequately considered. We have shown, for example, that the lid of a plastic urine collection jar contained more than 100 mg kg−1 Sb.3 Morever, a plastic dispenser commonly used to handle acids in the lab created a profound Sb contamination problem, with several μg l−1 Sb found in the dispensed HCl, compared with tens of ng l−1 Sb in the acid itself.3 As a result, it is at present unclear whether the reported values for Sb in bottled waters are accurate reflections of the abundance of Sb originally present in the fluid, or whether the measured Sb concentrations represent a contamination artefact. The release of Sb from plastics into fluids might have wider implications for the scientific communities studying environmental and health aspects of antimony.
In this context, PET (polyethylene terephthalate) is of particular relevance. A polyester of terephthalic acid and ethylene glycol, 90% of the PET manufactured worldwide employs Sb2O3 as a catalyst.9 Antimony trioxide is a suspected carcinogen, and is listed as a priority pollutant by the US EPA, the EU, and the German Research Foundation. Currently, an estimated 150 billion bottles are produced annually using PET. According to Nishioka et al.,10 some PET bottles used for drinks in Japan contain Sb, but others do not: he found that Sb concentrations were either in the range 170 to 220 mg kg−1 Sb, or they were below the limit of detection (< 0.1 mg kg−1). Although there have been several studies of Sb and other trace elements in bottled waters, no clear connection has yet been made between the abundance of Sb in the waters and the composition of the container materials, primarily because of inadequate detection limits,8,11 even when preconcentration is employed.12 Studies of Sb release from PET into food simulants using INAA13 suffered from high detection limits, but experiments employing ICP-QMS documented leaching of Sb from PET using 3% acetic acid at 40 °C for 10 days and 100 °C for 2 hours.14
Given that the natural abundance of Sb in groundwaters may be in the order of a few parts per trillion3 and our clean lab facilities present an opportunity to measure Sb at concentrations of parts per quadrillion,4 the quantitative determination of this element in bottled waters should provide a sensitive measure of the effects of the containers on the fluids. The main objective of this small study is to determine whether the elevated concentrations of Sb reported in bottled waters are simply a reflection of geological and mineralogical diversity of the source regions, or whether they have become contaminated by the bottles used to contain them.
Materials and methods
We purchased 15 popular brands of water bottled in PET containers from Canada as well as 48 from Europe: Germany (13), France (9), Switzerland (4), Finland (4), Czech Republic (4), Denmark (3), Spain (3), Poland (2), Belgium (2), The Netherlands (2), and Italy (2). Three of the brands from Germany were purchased both in PET and in glass bottles; these are denoted Brands A, B, and C. We also had the opportunity to collect water directly from the source of Brand A, prior to filtration and bottling, on July 18th, 2005. Wearing polyethylene gloves and a hair net, samples were collected directly into acid-cleaned, 100 ml low density polyethylene (LDPE) bottles to which high purity HNO3 (100 μl) had already been added. This acid is produced in-house, purified twice by sub-boiling distillation, and has an average Sb concentration of <0.03 ng l−1. Addition of 100 μl of this acid to 100 ml of pristine groundwater from Canada reduced the pH to 1.7 which is sufficient to stabilise the trace metals until the samples could be measured. To minimise the risks of contamination, none of the water samples were filtered. For comparison with these waters bottled in PET, a natural water from Ontario, Canada, bottled commercially in polypropylene, was analysed for comparison.Our LDPE bottles had been prepared in the same manner as the bottles which have successfully been used to measure trace elements in polar snow and ice.15,16 All cleaning procedures and sample manipulations were carried out in metal-free laminar flow clean benches of US class 100 with the operator wearing PE gloves. The 100 ml LDPE bottles and screw caps used for the collection of waters were initially rinsed five times with high purity water (18.2 MΩ cm−1) supplied from a MilliQ-Element system (Millipore, MA, USA). Thereafter the bottles were filled with 10% nitric acid for 3 weeks. This acid had been prepared in-house and was distilled twice by sub-boiling, using a commercial instrument made of high purity quartz (MLS, Leutkirch, Germany). Similarly, the screw caps were submerged into 10% HNO3 and left in the clean bench for 3 weeks, before both the bottles and the caps were again rinsed with high purity water and filled with 1% HNO3 for another week. Subsequently, the bottles and caps were rinsed again five times with high-purity water and dried in the clean bench overnight, before adding 100 μl high purity HNO3 to the bottles and sealing the bottles with the screw cap. For practical reasons and to reduce the risk of contamination during sampling, the acid was added to each bottle in the lab. Bottles containing acid were then packed individually in plastic bags, and sealed for transport to the field.
The average concentration of Sb in 15 independent blank solutions containing 0.5% HNO3 was 43 ± 10 pg l−1 and mainly reflects the contribution of Sb from the high purity water and not that of the acid. Analyses of different HNO3 concentrations (0.5%, 1%, 2%, 5%, 10%) produced comparable signals, i.e. increasing acid concentrations had no detectable influence on the Sb signal intensity. Therefore, Sb contributions from HNO3 are below the detection limit of 0.03 ng l−1.
Water samples collected from the source at Brand A were packed into three ziplock plastic bags and kept cool until they could be refrigerated in the laboratory in Heidelberg (ca. four hours later). Although these samples were not analysed until four months after collection, there was little risk of contamination during storage. Our previous measurements of Sb in groundwaters from Canada showed that even after six months of storage in the refrigerator, there was no detectable contamination of the waters by Sb from the acid-cleaned LDPE containers.3
All samples of bottled water were handled in metal-free Class 100 clean air cabinets. Antimony and other selected trace elements, including Pb and U, were determined in the waters using inductively coupled plasma-sector field mass spectrometry (ICP-SMS) applying ultra clean techniques as previously adapted for the determination of trace elements in polar ice.15,16 To this end, a tandem spray chamber arrangement including a low flow PFA nebulizer (ESI) operated in the self-aspirating mode, was employed. Details about instrument settings, acquisition and evaluation parameters are given elsewhere.17
For quality control purposes, SLRS-4, a certified, standard reference material (river water) produced by the National Research Council of Canada was analysed along with the samples. The measured concentrations of Sb (224 ± 12 ng l−1, n = 17) agreed well with the certified value (230 ± 40 ng l−1).
Results
Bottled waters from Canada
Compared to the natural abundance of Sb in pristine groundwaters from Springwater Township, Ontario (2.2 ± 1.4 ng l−1, n = 34), twelve brands of water from Canada, all in PET bottles, contained 112–375 ng l−1 (n = 21). Of these twelve brands, eight were bottled in the same region of southern Ontario as the ‘pristine groundwater’ described earlier.3 Given that southern Ontario consists of a series of sedimentary platforms, it seems unlikely that geological variation can explain these pronounced differences in Sb concentrations. Moreover, the three brands of deionised water bottled in PET from Ontario contained 134–195 ng l−1 Sb. Given that deionised water should contain very low concentrations of Sb (e.g. <0.1 ng l−1), the comparatively high concentrations of Sb in bottled deionised waters suggests that there is leaching of Sb from the bottles. Finally, the natural water from Ontario which is packaged in polypropylene (PP) bottles contains only 8.2 ± 0.7 ng l−1 (n = 7); this was the only bottled water found to contain concentrations of Sb comparable to the pristine groundwaters from southern Ontario. Taken together (Fig. 1), this data suggests that all of the waters bottled in PET containers, both the natural waters as well as deionised waters, have become contaminated with Sb leaching from their containers.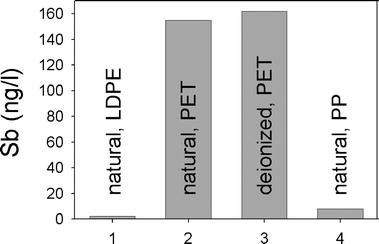 | ||
| Fig. 1 Antimony concentrations (ng l−1) in waters from Canada: pristine groundwater, Springwater Township, Simcoe County, Ontario,3 12 brands of natural water in PET containers, 3 brands of deionized water, and one brand of natural water bottled commercially in polypropylene. | ||
Bottled waters from Europe
The results of the Sb determinations of waters sold by Brands A, B, and C in Germany are given in Table 1. Water collected at the source of Brand A, prior to filtration and bottling, yielded 3.8 ± 0.9 ng l−1 Sb (n = 5), compared with the same brand of water purchased locally in PET bottles containing 359 ± 54 ng l−1 (n = 6). These six bottles of Brand A in PET were purchased a few days prior to measurement. However, this same brand of water in PET bottles, purchase three months earlier and stored at room temperature, yielded 626 ± 15 ng l−1 Sb (n = 3). These results show unambiguously that there is a profound leaching of Sb from the PET container into the water. The increase in Sb concentrations (75% during three months storage of Brand A), represents a leaching rate of approximately 100 ng l−1 per month. Analyses of Sb in water from Brand B and C, in glass bottles versus PET, supports this interpretation (Table 1). In the case of Brand C, the water bottled in PET contains approximately 10 times more Sb than the water stored in glass bottles. In the case of Brand A, the water bottled in PET contains 95 to 165 times more Sb than the original source water, depending on the time of storage. Moreover, the data suggests that the Sb concentration in the waters bottled in PET are independent of the natural abundance in the source water, but rather dependent on the time of reaction between the bottle and the fluid (i.e. the duration of storage).| Brand | Source | Glass | PET (purchased October, 2005) | PET (purchased July, 2005) |
|---|---|---|---|---|
| A | 3.8 ± 0.9 (n = 5) | 11.5 ± 4.4 (n = 6) | 359 ± 54 (n = 6) | 626 ± 15 (n = 3) |
| B | N/A | 84.5 ± 10.2 (n = 6) | 255 ± 20 (n = 6) | |
| C | N/A | 26.4 ± 3.1 (n = 6) | 301 ± 43 (n = 6) |
Five other brands of water from Germany bottled in PET contained 253–546 ng l−1 Sb (n = 5). Three other brands of water from Germany, bottled in plastic bottles which were not identified as PET, but rather as ‘recyclable’ contained only 32–60 ng l−1 Sb (n = 3). In this latter case, it may be supposed that either these bottles are made of a polymer or polymers which do not employ Sb as catalyst, or they had previously been recycled a sufficient number of times to have leached Sb out of the surface layers of the bottle walls.
With respect to the thirty-five brands of water in PET bottles from other countries in Europe (Fig. 2), the median Sb concentration is 343 ng l−1 (n = 35). The lowest concentration found (6 ng l−1) was in a sample from Poland, but based upon all of our data to date, we suspect that this bottle is either not made of PET, or it is made of PET manufactured without the use of Sb.9 The data shown in Fig. 2 indicates that the range in Sb concentrations is rather limited, with most values within a factor of two of the median. In contrast, our measurements of Pb and U in these same suite of samples show far greater variations: Pb varies from 0.7 to 1008 ng l−1 (more than 3 orders of magnitude) and U from 0.077 to 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 ng l−1 (more than 5 orders of magnitude). The large variations in the abundance of Pb and U in these waters are a reflection of the geological and mineralogical diversity of the source areas. The abundance of Sb in the waters, however, appears to be independent of geology.
550 ng l−1 (more than 5 orders of magnitude). The large variations in the abundance of Pb and U in these waters are a reflection of the geological and mineralogical diversity of the source areas. The abundance of Sb in the waters, however, appears to be independent of geology.
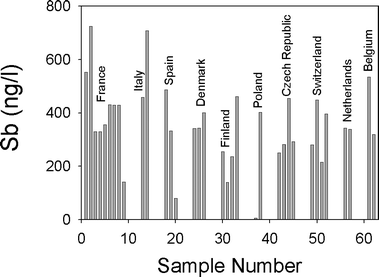 | ||
| Fig. 2 Antimony concentrations (ng l−1) in European mineral waters bottled in PET. The data for the water samples from Germany are given in Table 1 and in the text. | ||
Although there is a strong geochemical and mineralogical association between Pb and Sb at the surface of the earth,1 there is no correlation between the abundance of Sb in waters bottled in PET, and the abundance of Pb. We note further that the natural abundance of Pb to Sb in typical freshwaters is 15 ∶ 1.18 For comparison, our measurements of Pb and Sb in pristine groundwaters from Canada indicate that the ratio of Pb to Sb is approximately 10 ∶ 1 (Shotyk, unpublished). In the waters bottled in PET from the countries shown in Fig. 2, the median Pb concentration is 5 ng l−1. Assuming that the natural ratio of Pb to Sb in pristine groundwaters is ca. 10 ∶ 1, the median Pb concentration (5 ng l−1) implies that a median Sb concentration of 0.5 ng l−1 could reasonably be expected. However, the median Sb concentration in the European bottled waters is 343 ng l−1 Sb. These results and arguments imply that the waters bottled in PET have a much greater ratio of Sb to Pb, by many hundreds of times, compared to the ratio of their occurrence in nature. We assume, based on our measurements to date, that all of the waters bottled in PET, except one brand from Poland, have become contaminated with Sb leaching from the bottles.
To independently confirm that PET containers can have a significant effect on the Sb concentration in the fluids they contain, one of the PET bottles from Brand A was rinsed with pristine groundwater (containing 2.2 ± 1.2 ng l−1 Sb) at an artesian flow (Lot 5, Concession 10, Springwater Township, Ontario, Canada), allowed to leach for several days, and rinsed again with this water. Following the leaching and rinsing, it was filled with the pristine groundwater and shipped airfreight in a cool box to our lab in Germany. After only 37 days storage, in the refrigerator, this water was found to contain 59 ± 17 ng l−1 Sb (n = 2). In an earlier experiment using this approach, this same pristine water, after storage in the same brand of PET bottle at room temperature for six months, yielded 566 ng l−1 Sb (n = 1), an increase of more than 250 times.
Discussion
Contamination of bottled waters from Sb in PET versus glass containers
The data shown in Table 1 shows not only that water bottled in PET is contaminated with Sb, but also the water bottled in glass. Therefore, the natural abundance of Sb in groundwaters can neither be obtained from waters stored in PET nor in glass containers. Using instrumental neutron activation analyses (INAA), we found that a PET bottle for water (Brand A) and one for cola contained 397 and 351 mg kg−1 Sb, respectively; these very high concentrations certainly reflect the use of Sb2O3 as a catalyst in the manufacture of PET.9 However, we also used INAA to measure Sb in one glass bottle used for water (Brand A) and one for cola; these contained 7.6 and 10.1 mg kg−1 Sb, respectively. The presence of Sb in the glass bottles probably reflects its use as an opacifier in the manufacture of glass. Even in glass bottles, therefore, some leaching of Sb into waters has to be expected. Although our data suggests that leaching of Sb from PET is far greater than from glass, our study included 48 brands of water in PET, but was limited to only 3 brands in glass. No general conclusions about the extent of leaching of Sb into waters from glass bottles should be made based on the limited number of samples considered in the present study.Factors affecting the leaching of Sb from the PET containers
The results presented here give rise to many questions regarding the chemical and mineralogical forms of Sb in PET containers, spatial variation in Sb concentrations within these polymers, and the release of Sb to bottled waters and other beverages. For example, what is the relationship between the concentration of Sb in the polymer and its rate of release to the water? How does this rate vary with the pH of the water, temperature, presence of other cations and anions, storage conditions, and reaction time? How much of the Sb in the beverage is in the form of Sb(III) and how much Sb(V)? With respect to acidic drinks (vinegar, fruit juices, cola, lemon juice) what is the effect of pH on the Sb release rate? What is the effect of citric acid and other organic ligands on the release rate? Also, what is the effect of conditioning of the bottles by washing during recycling and reuse?Conclusions
The data presented here leave little doubt that bottled waters stored in PET are contaminated with Sb from their containers. The motivation for our study has been to demonstrate that bottled waters cannot be used to study the natural abundance of Sb in groundwaters. Moreover, Sb is widely used in plastics commonly found in many laboratories. We suspect that sample contamination by Sb-bearing containers and sample handling equipment is more widespread than generally realised. We note further that PET is used not only for drinks bottles, but also for filtering beverage products, food packaging, and in the pharmaceuticals industry.We wish to emphasise that all of the waters measured in our lab to date were found to contain Sb in concentrations well below the guidelines commonly recommended for drinking water which are as follows: WHO, 20 ug l−1; US EPA, Health Canada and the Ontario Ministry of Environment, 6 ug l−1; German Federal Ministry of Environment, 5 ug l−1; Japan, 2 ug l−1. However, given that there appears to be a continual release of Sb from the containers to the fluids, and that the Sb concentrations in the waters mainly reflect the duration of storage, systematic studies of the extent and intensity of contamination are warranted.
Acknowledgements
We are grateful to Stefan Rheinberger and James Zheng for technical support, and to Andriy Cheburkin for helpful discussions. Emma and Olivia Shotyk helped with the field work in Germany. We thank the following friends and colleagues for providing bottles of water: Gaël Le Roux, Tommy Nørnberg, Kimmo Virtanen, and Henrik Wild.References
- W. Shotyk, M. Krachler and B. Chen, in The Biogeochemistry and Cycling of Antimony, Biogeochemistry, Availability, and Transport of Metals in the Environment, vol. 44, Metal Ions in Biological Systems, ed. A. Sigel, H. Sigel and R. K. O. Sigel, M. Dekker, New York. 2005, pp. 172–203 Search PubMed.
- R. W. Boyle and I. R. Jonasson, J. Geochem. Explor., 1984, 20, 223 CrossRef.
- W. Shotyk, M. Krachler, B. Chen and J. Zheng, J. Environ. Monit., 2005, 7, 1238 RSC.
- M. Krachler, J. Zheng, C. Zdanowicz, R. Koerner, D. Fisher and W. Shotyk, J. Environ. Monit., 2005, 7, 1169 RSC.
- R. W. Dabeka, H. B. S. Conacher, J. F. Lawrence, W. H. Newsome, A. McKenzie, H. P. Wagner, R. K. H. Chadha and K. Pepper, Food Addit. Contam., 2002, 19, 721 CrossRef CAS.
- A. Misund, B. Frengstad, U. Siewers and C. Reimann, Sci. Total Environ., 1999, 244, 21 CrossRef.
- B. Frengstad, D. Banks and U. Siewers, Sci. Total Environ., 2001, 277, 101 CrossRef CAS.
- J. Naohara, Mizu Kankyo Gakkaishi, 1998, 21, 536 Search PubMed (in Japanese, Chem. Abstr., 130:7173).
- U. K. Thiele, Chem. Fibers Int., 2004, 54, 162 Search PubMed.
- K. Nishioka, A. Hirahara and E. Iwamoto, Bull. Inst. Life Sci. Hiroshima Prefectural Women's Univ., 2002, 8, 35 Search PubMed.
- J. Suzuki, Y. Katsuki, H. Ogawa, K. Suzuki, H. Matsumoto and K. Yasuda, Shokuhin Eiseigaku Zasshi, 2000, 41, 387 Search PubMed (in Japanese, Chem. Abstr., 134:120402).
- K.-H. Lee, M. Oshima and S. Motomizu, Bunseki Kagaku, 2000, 49, 529 CrossRef CAS (in Japanese, Chem. Abstr., 133:155011).
- D. Thompson, S. J. Parry and R. Benzing, J. Radioanal. Nucl. Chem., 1996, 213, 349 CAS.
- P. J. Fordham, J. W. Gramshaw, H. M. Crews and L. Castle, Food Addit. Contam., 1995, 12, 651 CAS.
- M. Krachler, J. Zheng, D. Fisher and W. Shotyk, J. Anal. At. Spectrom., 2004, 19, 1017 RSC.
- M. Krachler, J. Zheng, D. Fisher and W. Shotyk, Anal. Chim. Acta, 2004, 530, 291.
- M. Krachler, N. Rausch, H. Feuerbacher and P. Klemens, Spectrochim. Acta, Part B, 2005, B60, 865 CrossRef.
- H. J. M. Bowen, Environmental Chemistry of the Elements, Academic Press, New York, 1979 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |
