Road pavers’ occupational exposure to asphalt containing waste plastic and tall oil pitch†
Virpi
Väänänen
*,
Eivor
Elovaara
,
Erkki
Nykyri
,
Tiina
Santonen
and
Pirjo
Heikkilä
Finnish Institute of Occupational Health, Topeliuksenkatu 41 aA, FI-00250, Helsinki, Finland. E-mail: virpi.vaananen@ttl.fi; Fax: +358 30 474 2114; Tel: +358 30 474 2809
First published on 29th November 2005
Abstract
Waste plastic (WP) and tall oil pitch (T), which are organic recycled industrial by-products, have been used as a binder with bitumen in stone mastic asphalt (SMA) and asphalt concrete (AC). We compared the exposure over one workday in 16 road pavers participating in a survey at four paving sites, using mixes of conventional asphalt (SMA, AC) or mixes containing waste material (SMA-WPT, AC-WPT). The concentrations of 11 aldehydes in air were 515 and 902 μg m−3 at the SMA-WPT and AC-WPT worksites, being 3 and 13 times greater than at the corresponding worksites laying conventional asphalt. Resin acids (2–42 μg m−3), which are known sensitizers, were detected only during laying of AC-WPT. The emission levels (μg m−3) of total particulates (300–500), bitumen fumes (60–160), bitumen vapour (80–1120), naphthalene (0.59–1.2), phenanthrene (0.21–0.32), pyrene (<0.015–0.20), benzo(a)pyrene (<0.01) and the sum of 16 PAHs (polycyclic aromatic hydrocarbons, 1.28–2.00) were similar for conventional and WPT asphalts. The dermal deposition of 16 PAHs on exposure pads (on workers’ wrist) was low in all pavers (0.7–3.5 ng cm−2). Eight OH-PAH biomarkers of naphthalene, phenanthrene and pyrene exposures were quantified in pre- and post-shift urine specimens. The post-shift concentrations (mean ± SD, μmol mol−1 creatinine) of 1- plus 2-naphthol; 1-,2-,3-,4- plus 9-phenanthrol; and 1-hydroxypyrene were, respectively, for asphalt workers: 18.1 ± 8.0, 2.41 ± 0.71 and 0.66 ± 0.58 (smokers); 6.0 ± 2.3, 1.70 ± 0.72 and 0.27 ± 0.15 (non-smokers); WPT asphalt workers: 22.0 ± 9.2, 2.82 ± 1.11 and 0.76 ± 0.18 (smokers); 6.8 ± 2.6, 2.35 ± 0.69 and 0.46 ± 0.13 (non-smokers). The work-related uptake of PAHs was low in all pavers, although it was significantly greater in smokers than in non-smokers. The WPT asphalt workers complained of eye irritation and sore throat more than the pavers who had a much lower exposure to aldehydes and resin acids.
 Virpi Väänänen Virpi Väänänen | Virpi Väänänen (MSc, Lic) was born in Finland, in 1966. In 1997 she joined the Finnish Institute of Occupational Health, Department of Industrial Hygiene and Toxicology as a chemist and is studying for her doctoral thesis at the University of Kuopio. |
Introduction
Road pavers are exposed not only to the emissions from the newly-laid asphalt, but also to exhaust gases from the passing traffic and paving machines. Since the 1990s the occupational exposure of the road pavers has been studied in Scandinavia and USA.1–12 The inhalation exposure of the pavers has been low compared to the occupational exposure limits (OEL), in general, but significantly greater than for example the exposure of traffic controllers who work at a location near to the pavers. New concerns arise in conjunction with some new applications, such as recycling of the old asphalt and the use of industrial by-products in asphalt pavements.13 Asphalt consists of bitumen (4–8% w/w) which is a by-product of crude oil refining, fillers (0–10% w/w), fibres (0–4% w/w) and crushed stones. Bitumen is used to bind the filler and gravel. Fillers and fibres are added to modify the properties of the asphalt.There are only a few published studies assessing the exposure of the workers during the paving with asphalt mixtures containing industrial by-products or waste materials.2,9,14,15
The National Institute for Occupational Safety and Health (NIOSH) has studied the occupational exposure of asphalt workers during the laying of crumb rubber modified (CRM) asphalt. The breathing zone air impurities (total particulates, benzene soluble particulates, PAHs, organic sulfur-containing compounds) were higher during CRM asphalt paving than during paving with conventional asphalt.2 Also in the study of Paananen, the air concentrations of total particulates, bitumen fumes and PAHs were increased when asphalt was modified with waste plastic and tall oil pitch in comparison with conventional asphalt.15 We have shown by means of ambient, dermal and biological monitoring that the addition of an inorganic industrial by-product, coal fly ash as the filler into SMA, did not affect the pavers’ exposure to airborne PAHs nor the level of PAH metabolites found in their urine.8 Repaving operations have been reported to increase respiratory PAH exposure in paving crews.16 According to our earlier studies, repaving workers excreted a higher level of OH-PAH metabolites in their urine than the ordinary paving workers.8 Also, the dermal exposure of the workers was higher during remixing than during SMA paving.9 The hot recycling in situ and the use of waste plastic in bitumen were stated to increase the mutagenicity of the fumes in the Ames test compared to the SMA paving fumes and bitumen fumes generated in laboratory.17 In these previous studies, the waste plastics (WP) contained, e.g., polyvinyl chloride polymers.15,17 In this study, waste plastics composed only of polyethylene and polypropylene. Tall oil pitch is the residue remaining after distillation of tall oil which is a by-product from the pulp industry. Tall oil pitch is composed of free acids, esterified acids and unsaponifiable neutral compounds. The low molecular weight free acids are mainly dehydroabietic, abietic and other resin acids.18
The International Agency for Research on Cancer (IARC) has evaluated the carcinogenicity of bitumen in 1985 and 1987.19,20 This health risk is based on the fact that asphalt/bitumen fumes contain low concentrations of benzo(a)pyrene (BaP) and other known PAH carcinogens.21,22 The latest cohort study of European asphalt workers provides some evidence of bitumen fume carcinogenicity after adjustment for coal tar exposure.23,24 Asphalt work is also associated with irritation of eyes and airways.2,22,25,26 The intensity of irritation increases with increasing asphalt temperature and with increasing concentrations of asphalt fumes.25
As a continuation of our studies on inorganic recycled additives in asphalts,8,9,17 in this survey we studied the occupational exposures, when organic recycled industrial by-products were mixed in asphalt. The external exposure was studied by measuring the concentrations of various airborne impurities and the dermal deposition of PAHs by using wrist-mounted exposure pads. The internal exposure (total uptake) was monitored by measuring the concentration of eight OH-PAH metabolites in urine samples. The symptoms experienced by the workers were surveyed by a questionnaire.
Materials and methods
Subjects and study design
The exposure of asphalt workers was surveyed at four paving sites in July 2003 during the laying of the following asphalt mixtures: AC, AC-WPT, SMA and SMA-WPT. The SMA-paving sites were located in a city area and the AC-paving sites were in suburban/rural areas. The mixtures were laid at temperatures of 145–165 °C. The weather was warm (18–28 °C) and clear at all paving sites. The Finnish Plastics Industries Federation organized the waste plastic (polyethylene 90% and polypropylene 10%) from Muovix (Riihimäki, Finland) and Arizona Chemical donated the tall oil pitch (Oulu, Finland). The content of asphalt and the laying temperatures are shown in Table 1.| Asphalt mixture | Binder (w-%) | Fiber (w-%) | Filler (w-%) | Stones (w-%) | Temperature of newly laid asphalt/°C |
|---|---|---|---|---|---|
| AC-WPT | 5.3 binder: | ||||
| 70 bitumen | |||||
| (B200) | — | 2.0 lime | 90.7 | 165 | |
| 18 tall oil pitch | 2.0 coal fly ash | ||||
| 12 waste plastic | |||||
| AC | 5.3 bitumen | — | 2.0 lime | 90.7 | 145 |
| (B80) | 2.0 coal fly ash | ||||
| SMA-WPT | 6.1 binder: | 0.3 cellulose | 11.0 lime | 82.6 | 151 |
| 70 bitumen (B200) | |||||
| 18 tall oil pitch | |||||
| 12 waste plastic | |||||
| SMA | 5.9 bitumen (B80) | 0.3 cellulose | 11.0 lime | 82.2 | 157 |
| 0.6 natural asphalt |
Sixteen male asphalt workers (6 non-smokers and 10 smokers, age 18–59 years) and 2 female traffic controllers (smokers, 19–20 years age) participated on one, two, or four days in this field study. Paving teams consisted of 7–10 members with tasks described by the following job titles, i.e. paver operator, screed man, shovelers/rakermen, roller operators and traffic controllers. The paver operator controls the paving machine, sitting on the top of the vehicle. The screed man operates the paving screed to the desired dimensions and stands at the rear of the paving machine. Shovelers and rakermen manually spread the asphalt with hand tools such as rakes and shovels. Roller operators drive the rolling machine to compact the newly paved asphalt. Traffic controllers guide the passing traffic from a distance farther away from the paving site and served thus as reference subjects having a minimum of ambient and dermal exposure to PAHs from asphalt mixtures. The numbers of the participating paving workers and the collected samples are presented in Table 2.
| Paving site | Air samples | Skin samples | Urine samples | Sampling day |
|---|---|---|---|---|
| a AC-WPT = asphalt concrete containing waste plastic and tall oil pitch, AC = asphalt concrete, SMA-WPT = stone mastic asphalt, containing waste plastic and tall oil pitch, SMA = stone mastic asphalt. b In parentheses, sample number from traffic controllers. | ||||
| AC-WPT | 5 (1)b | 4 (1)b | 6 (1)b | Tuesday |
| AC | 5 (1)b | 4 (1)b | 10 (1)b | Monday |
| SMA-WPT | 5 | 5 | 7 | Thursday |
| SMA | 5 | 5 | 7 | Monday |
| Samples (n) from road pavers (n = 9–16) | 20 (9) | 18 (10) | 30 (16) | |
| Samples (n) from traffic controllers (n = 2) | 2 | 2 | 2 | |
The paving machines were not equipped with a cabin or a ventilation system. The asphalt workers did not wear respiratory masks or use any barrier creams, but most of them did wear gloves. The workers could not wash their hands or take a shower at the paving sites.
Application of WPT asphalt was a pilot project. Laying days were decided by the contractor and therefore we were not able to choose the weekday of the sampling (Table 2).
Air sampling and analysis
We measured the concentrations of total particulates, bitumen fume and vapour, 16 PAHs (listed by EPA (Environmental Protection Agency) standard), four methylated PAHs, 11 aldehydes and resin acids at all of the paving sites. Air samples were collected at the breathing zone of the worker or as near to the working area as possible. The sampling periods for particulates, bitumen fume and vapour and PAHs were four hours during laying of SMA and SMA-WPT and eight hours during laying of AC and AC-WPT. We collected particulates, bitumen fumes and vapours and PAHs with the closed faced 37 mm samplers connected to the XAD-adsorbent and used the sampling rate of 1.0–1.5 ml min−1.Particulates and bitumen fumes were sampled on Teflon filters (SKC 225-17-05) that were air-conditioned (relative humidity 50 ± 5) before and after sampling for weighing. The quantitation limit for the gravimetric method was 0.1 mg. After the weighing, filters were extracted with tetrachloroethylene (Merck, p.a., 2 ml) in an ultrasonic bath (30 min) and analyzed in a Fourier transform infrared spectrometer (FTIR).3
Bitumen vapours were eluted from the XAD-2-adsorbent (SKC 226-30-06) with dichloromethane (Merck, p.a., 2 ml) in an ultrasonic bath and analyzed with gas chromatography with a flame ionisation detector (GC-FID) according to the in-house method (Finnish Institute of Occupational Health (FIOH), Uusimaa Regional Institute).3
PAH compounds collected on Teflon filters (SKC 255–1707) connected with XAD-2 tubes (Supelco Orbo-43, Cat. No 20258) were extracted with cyclohexane (Merck, p.a.) and dichloromethane (Merck, SupraSolv) mixture (4 + 1, 10 ml for filters and 4 ml for XAD-2 adsorbents) in an ultrasonic bath. An internal standard mixture, that contained six deuterated PAH compounds, was added into the sample before the extraction.9 The extracts of the filters were purified with liquid–liquid partition and back extraction and with solid-phase extraction.27,28 In the first stage of the purification, the extract (10 ml) was evaporated down to a volume of 5 ml under the gentle flow of nitrogen in a water bath (TurboVap® LV Evaporator, Zymark). In a separating funnel, a mixture (9 + 1, 5 ml) of N,N-dimethylformamide (DMF, Rohmil, Super Purity Solvent) and MilliQ-water was added, shaken and the lower fraction was recovered. This procedure was done twice. To the recovered fraction, 10 ml MilliQ-water and 20 ml cyclohexane were added, shaken and the fraction of cyclohexane was recovered. Subsequently, the sample was evaporated to the minimum volume and transferred to the hexane and cyclohexane conditioned solid phase tube (Mega Bond Elut Si, 6 ml, Varian). PAH compounds were eluted with a mixture of cyclohexane and dichloromethane (9 + 1, 8 ml). Finally, the sample was evaporated to a volume of 300 μl and analyzed with gas chromatography equipped with a mass selective detector (GC-MSD, Agilent Technologies 6890N/5973N, Palo Alto, CA, USA) using selective ion monitoring (SIM). The analytical column was HP-5MS (30 m × 0.32 mm × 0.25 μm phase, J&W Scientific, Agilent Technologies). The temperature of the injector was 280 °C and the program of the oven was as follows: 50 °C (1 min) → 5 °C min−1→ 200 °C → 8 °C min−1 → 300 °C (10 min).9
The volatile PAH compounds were extracted from the XAD-adsorbent. After extraction, the samples were centrifuged (3000 rpm, 15 min), evaporated to a volume of 500 μl, cyclohexane was added to a final volume of 2 ml and analyzed with GC-MSD.
The sampling period for aldehydes and resin acids was 1–4 hours. Airborne aldehydes (acrolein, formaldehyde, acetaldehyde, benzaldehyde, hexanal, butanal, pentanal, crotonaldehyde, propionaldehyde, methylbenzaldehyde, 2-methylbutanal) and acetone were collected with the 2,4-dinitrohydrazine coated Sep-Pak samplers The aldehydes and acetone were eluted from the samplers with acetonitrile (Merck, HPLC-grade) and detected with HPLC using a diode arrow detector (DAD, 360 nm) according the EPA-standard method (FIOH, Uusimaa Regional Institute).29 The limit of quantification was 0.1 μg, which is 0.5 μg m−3 with a sample of 200 dm3. Resin acids were collected with an open faced sampler (37 mm) using acetone-prewashed class fibre filters. Resin acids were extracted with a diethylether–methanol mixture (9 + 1, 3 ml) and the compounds were derivatised with tetramethylammomiunhydroxide (0.1% v/v). The derivatisation reaction was slightly modified from a method for esterification of fatty acids.30 Isopimar acid, dehydroabietic acid, heptadecan acid and heinecosan acid were used as standards. The analysis was performed with GC/MSD according to the in-house method (FIOH, Turku Regional Institute) and the identification of the compounds was based on the Wiley library and colophony-reference material. The limit of detection was 0.5 μg sample−1, which is 1.3 μg m−3 with a sample of 400 dm3.
Dermal sampling and analysis
PAH compounds were determined from two pad samplers which were placed on the back of the wrists and worn by the workers over the whole shift. The sampling method and the sampling material are described elsewhere.9 The exposure pads were analyzed for 16 unalkylated PAH and four methylated PAH compounds with the same method as described above for the filters used to collect airborne particulate PAHs.Urinary PAH metabolites
Eight mono-hydroxylated PAH metabolites (OH-PAH) were determined by an HPLC-FLD method 31 as described for simultaneous quantification of 1- and 2-naphthol, 1-,2-,3-,4- and 9-phenanthrol and 1-OHP in human urine.32 Briefly, the urine samples were enzymatically deconjugated at 38 °C for 4 h with β-glucuronidase/arylsulfatase (from Helix pomatia, Roche Diagnostics GmbH, Mannheim, Germany) and prepared for analysis in a Shimadzu HPLC /CLASS VP 6.12 SP2 chromatography system with an RF-10Axl fluorescence detector (Shimadzu, Kyoto, Japan). The detection limits were low for 1-naphthol (6 nmol l−1 urine), 2-naphthol (2 nmol l−1), 1-,2-,3-,4-,9-OHPhe (<1 nmol l−1) and OHP (<1 nmol l−1). Quantitation was based on peak area (or the peak height in the case of 1-phenanthrol), using for calibration commercial reference compounds: the phenanthrols were from Dr Ehrenstorfer GmbH, Augsburg, Germany; 1-naphthol, 2-naphthol and 1-OHP were from Sigma-Aldrich Chemie GmbH, Steinheim, Germany. The recovery of naphthols, phenanthrols and 1-OHP added in control urine was good (93–103%). A PAH-positive urine (human) was run in each series as a quality control specimen.Questionnaire on the workers’ symptoms
The paving workers were interviewed using a standard questionnaire form. There were 14 workers interviewed at the four worksites. The questions in the questionnaire dealt with wide ranging issues, e.g., eye, skin and respiratory irritation, staining, odour, smoking and the use of protective clothing.Statistical methods
The differences in the air impurities, the PAH deposition on the exposure pads and the urinary metabolite concentrations between conventional asphalt workers and WPT asphalt workers were evaluated using the t-test for two independent samples. The association between the answers of the questionnaire and the measurements of the exposure (for example the airborne PAH concentration) were assessed by testing the difference of the means of the measurements in the classes of questionnaire using the t-test for two independent samples, as well. The differences between pre- and post-shift urine samples were tested using the paired t-test. Pearson’s correlation coefficients (r) were calculated to examine the association between the air impurity concentrations, the PAH deposition on the skin and the PAH metabolites in the urine samples. If the distribution of the values was skewed, logarithmic transformation was performed. Measurements below the detection limit were replaced by values of half of the limit of quantification (LOQ).Results
Air impurities
The use of waste plastic and tall oil pitch in asphalt had minor or no statistically significant effects on total particulates (p = 0.14), bitumen fume (p = 0.05) and vapour (p = 0.6) levels. The average exposures of the paving crews paving with AC, AC-WPT, SMA and SMA-WPT were similar at the breathing zones of the paving workers at all four paving sites (Table 3).| Asphalt mixture | Study performed in | N | Total particulates/mg m−3 | Bitumen fume/mg m−3 | Bitumen vapour (TVOC)/mg m−3 | N | Naphthalene/μg m−3 | Phenanthrene/μg m−3 | Pyrene/μg m−3 | BaP/μg m−3 | Total PAH/μg m−3 | Methylated PAH/μg m−3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a AM = arithmetic mean, GM = geometric mean, Total PAH = sum of 16 unalkylated PAHs, BaP = benzo(a)pyrene, LOQ = limit of quantification. b Data from our previous study Väänänen et al. 2003 and 2005.8,50 c Data from our previous study Heikkilä et al. 1994.34 | |||||||||||||
| AC-WPT | 2003 | AM | 0.4 | 0.16 | 1.1 | 0.59 | 0.28 | 0.019 | 1.28 | 0.44 | |||
| 5 | GM | 0.4 | 0.13 | 1.0 | 4 | 0.48 | 0.24 | 0.017 | 1.08 | 0.35 | |||
| Min–max | <LOQ–0.8 | 0.07–0.29 | 0.4–1.9 | 0.22–0.96 | 0.13–0.46 | <0.015-0.028 | <0.01 | 0.54–2.01 | 0.18–0.75 | ||||
| AC | 2003 | AM | 0.5 | 0.06 | 0.9 | 0.89 | 0.37 | 0.02 | 1.75 | 0.42 | |||
| 5 | GM | 0.4 | 0.06 | 0.8 | 4 | 0.87 | 0.36 | 0.018 | 1.69 | 0.40 | |||
| Min–max | 0.2–1.1 | 0.05–0.08 | 0.5–1.4 | 0.63–1.10 | 0.25–0.57 | 0.015–0.038 | <0.01 | 1.19–2.32 | 0.29–0.67 | ||||
| SMA-WPT | 2003 | AM | 0.4 | 0.14 | 0.8 | 0.78 | 0.21 | <0.015 | 1.33 | 0.18 | |||
| 5 | GM | 0.4 | 0.14 | 0.8 | 5 | 0.73 | 0.19 | <0.015 | 1.25 | 0.14 | |||
| Min–max | <LOQ–0.9 | 0.10–0.16 | 0.7–1.0 | 0.45–1.28 | 0.10–0.32 | <0.015–0.030 | <0.01 | 0.77–2.10 | 0.046–0.38 | ||||
| SMA | 2003 | AM | 0.3 | 0.15 | 0.9 | 1.20 | 0.32 | 0.017 | 2.00 | 0.29 | |||
| 5 | GM | 0.3 | 0.14 | 0.8 | 5 | 0.99 | 0.29 | <0.015 | 1.74 | 0.27 | |||
| Min–max | <LOQ–0.5 | 0.10–0.22 | 0.6–1.5 | 0.38–2.22 | 0.18–0.49 | <0.015–0.032 | <0.01 | 0.82–3.45 | 0.18–0.47 | ||||
| All pavers | 2003 | AM | 0.4 | 0.13 | 0.9 | 0.88 | 0.29 | 0.017 | 1.60 | 0.32 | |||
| 20 | GM | 0.4 | 0.11 | 0.9 | 18 | 0.75 | 0.26 | <0.015 | 1.42 | 0.26 | |||
| Min–max | <LOQ–1.1 | 0.05–0.29 | 0.4–1.9 | 0.22–2.2 | 0.10–0.57 | <0.015–0.038 | <0.01 | 0.54–3.45 | 0.046–0.75 | ||||
| Traffic controllers | 2003 | AM | 0.049 | 0.018 | 0.14 | ||||||||
| — | GM | — | — | — | 2 | 0.049 | 0.017 | 0.14 | |||||
| Min–max | 0.044–0.055 | 0.015–0.020 | <0.015 | <0.01 | 0.13–0.14 | <0.04 | |||||||
| SMAb | 1999–2000 | AM | 2.0 | 0.93 | 3.6 | 2.7 | 3.9 | 0.14 | 0.04 | 7.7 | |||
| 26 | GM | 1.1 | 0.42 | 1.7 | 21 | 2.1 | 2.5 | 0.06 | 0.02 | 5.5 | — | ||
| Min–max | 0.1–9.7 | 0.04–6.9 | 0.20–21 | 0.59–13 | 0.46–27 | 0.01–1.2 | <0.01–0.22 | 1.4–46 | |||||
| ACc | 1993 | AM | 0.44 | 0.08 | 4.0 | 2.4 | 0.43 | 0.35 | 4.0 | ||||
| 8 | GM | 0.42 | 0.08 | 2.7 | 12 | 1.9 | 0.27 | 0.26 | 3.2 | — | |||
| Min–max | 0.3–0.7 | 0.05–0.1 | 0.3–8 | 0.75–5.4 | 0.02–1.2 | 0.02–0.7 | <0.01 | 0.84–8.3 | |||||
| Remixing of AC or SMAb | 1999–2000 | AM | 1.1 | 0.26 | 1.2 | 4.0 | 2.1 | 0.14 | 0.08 | 9.0 | |||
| 16 | GM | 0.9 | 0.18 | 0.7 | 14 | 2.5 | 1.4 | 0.07 | 0.03 | 5.9 | — | ||
| Min–max | 0.26–1.9 | 0.02–0.64 | 0.2–5.2 | 0.28–10 | 0.20–4.5 | 0.01–0.44 | <0.01–0.32 | 0.87–24 | |||||
The air concentrations of 16 unalkylated PAHs and four methylated PAHs (Table 3) were slightly but statistically not significantly higher in the emissions of the conventional asphalt than was the case with the WPT asphalt (p > 0.05). Naphthalene (55 wt% of the total PAH amount), phenanthrene (18 wt%) and fluorene (12 wt%) were the most abundant unalkylated PAHs. Over 97 wt% of the PAHs (mainly with two or three aromatic rings) was found in the vapour phase and less than 3 wt% in the particulate phase. Pyrene and BaP could not be quantified in 50% and 100% of the air samples, respectively. The sum of the 16 PAHs measured at the breathing zone of the paving workers was on average of 1.6 μg m−3. This value was about ten times greater than the concentrations in the traffic controllers. The air concentration of the four methylated PAHs was approx. 20% (0.3 μg m−3) of the 16 unalkylated PAHs. The main methylated PAH compounds were 1-methylphenanthrene (38 wt% of the total methylated PAH amount), 2,10- and 4,10-dimethylphenanthrenes (54 wt%).
Airborne aldehydes and resin acids were collected on the top or at the rear of the paving machine. The concentration of 11 aldehydes in air was 515 and 902 μg m−3 at the SMA-WPT and AC-WPT worksites, being 3- and 13-fold greater than those measured at the corresponding paving sites laying conventional asphalt. The most abundant aldehydes were aliphatic aldehydes, benzaldehyde and their methylated derivatives. Airborne hexanal (80-fold), 2-methylbutanal (60-fold), benzaldehyde (58-fold), crotonaldehyde (40-fold), pentanal (43-fold) and methylbenzaldehydes (32-fold) were all increased in the emissions of AC-WPT. The amount of acetone was high in the emissions of SMA-WPT when compared to the other paving sites. Resin acids were detected only during laying of AC-WPT. The concentration of resin acids was 2 μg m−3 on the paving machine with sampling conducted near to the breathing zone of the paving operator and 42 μg m−3 at the rear of the paving machine, which was near to the breathing zone of the screed man. Dehydroabietic acid was the main resin acid in both samples, but abietic, pimar, isopimar and 6-dehydroabietic acids were also detected in the sample at the rear of the paving machine. Resin acids were not detected from the other samples collected during laying of AC, SMA and SMA-WPT. The amounts of airborne aldehydes, acetone and resin acids are presented in Table 4.
| Asphalt mixture | Number of samples | Acrolein | Crotonaldehyde | Formaldehyde | Acetaldehyde | Propanal | n-Butanal | Pentanal | Hexanal | 2-Meth. butanal | Benzaldehyde | Meth. benzaldehydes | Acetone (ketone) | The sum of the aldehydes | Resin acids | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Sampler was overloaded, the actual concentration might be more than the written concentration. AM = arithmetic mean, SD = standard deviation. LOD = limit of detection (1.3 μg m−3 with a sample of 400 dm3). | ||||||||||||||||
| AC-WPTa | 2 | AM | 5.6 | 12.5 | 47 | 85 | 49 | 81 | 172 | 316 | 18 | 106 | 9.8 | 1.4 | 902 | 22 |
| SD | 0.3 | 0.3 | 1.9 | 18 | 7.2 | 0.1 | 14 | 33 | 1.7 | 7.1 | 0.6 | 0.3 | 33 | 28 | ||
| AC | 2 | AM | <0.6 | <0.6 | 9.3 | 32 | 8.0 | 5.7 | 4.0 | 4.0 | <0.6 | 1.8 | <0.6 | 17 | 65 | <LOD |
| SD | 1.6 | 4.9 | 0.5 | 0.4 | 0.4 | 0.9 | 0.4 | 2.3 | 9.2 | |||||||
| SMA-WPT | 2 | AM | <1.5 | 5.0 | 34 | 111 | 42 | 45 | 76 | 150 | <1.5 | 47 | 5.1 | 181 | 515 | <LOD |
| SD | 2.2 | 2.6 | 53 | 21 | 22 | 42 | 84 | 25 | 1.0 | 110 | 254 | |||||
| SMAa | 2 | AM | <0.7 | 2.0 | 19 | 52 | 19 | 17 | 14 | 15 | <0.7 | 2.9 | <0.7 | 22 | 140 | <LOD |
| SD | 0.5 | 4.3 | 14 | 4.7 | 4.5 | 1.8 | 4.2 | 0.7 | 3.8 | 34 | ||||||
Dermal contamination
The dermal PAH contamination of the paving workers was monitored with the wrists pads during the whole workshift and the results are presented in Table 5. The amounts of 16 unalkylated PAHs and the methylated PAHs were slightly but statistically not significantly (p > 0.05) higher in the pad samplers of the conventional asphalt workers than in those of the WPT asphalt workers. The deposits of 16 unalkylated PAHs (AM 1.6 ng cm−2) were less than that of methylated PAHs (AM 3.1 ng cm−2) on the pads of the asphalt workers. The same unalkylated and methylated PAH compounds were predominant on the skin and in the breathing zone air. On the skin pads, we could not detect any PAH compound with more than four aromatic rings.| Asphalt mixture | N | Naphthalene | Phenanthrene | Pyrene | Total PAH | Methylated PAH | |
|---|---|---|---|---|---|---|---|
| a AM = arithmetic mean, GM = geometric mean, total PAH = sum of 16 unalkylated PAHs. b Data from our previous study, Väänänen et al. 2005.9 | |||||||
| AC-WPT | AM | <0.08 | 0.11 | <0.09 | 0.86 | 2.3 | |
| 4 | GM | <0.08 | <0.08 | <0.09 | 0.85 | 2.1 | |
| Min–max | <0.08–0.19 | <0.08–0.32 | <0.09 | 0.71–1.1 | 1.2–3.5 | ||
| AC | AM | 0.84 | 0.32 | 0.37 | 1.8 | 3.7 | |
| 4 | GM | <0.08 | 0.22 | 0.34 | 1.7 | 3.7 | |
| Min–max | <0.08–0.22 | <0.08–0.60 | 0.18–0.48 | 1.2–2.4 | 3.1–4.5 | ||
| SMA-WPT | AM | 0.27 | 0.28 | 0.11 | 1.4 | 1.5 | |
| 5 | GM | 0.12 | 0.19 | <0.09 | 1.2 | 1.4 | |
| Min–max | <0.08–0.64 | <0.08–0.63 | <0.09–0.25 | 0.83–2.4 | 0.84–2.1 | ||
| SMA | AM | 0.35 | 0.68 | 0.32 | 2.4 | 4.4 | |
| 5 | GM | 0.23 | 0.46 | 0.20 | 2.1 | 3.4 | |
| Min–max | <0.08–0.69 | <0.08–1.1 | <0.09–0.47 | 1.1–3.5 | 1.0–10 | ||
| All pavers | AM | 0.22 | 0.34 | 0.19 | 1.6 | 3.1 | |
| 18 | GM | 0.11 | 0.19 | 0.12 | 1.4 | 2.5 | |
| Min–max | <0.08–0.69 | <0.08–1.1 | <0.09–0.48 | 0.71–3.5 | 0.84–10 | ||
| Traffic controllers | AM | ||||||
| 2 | GM | ||||||
| Min–max | <0.08 | <0.08 | <0.09 | <0.66–0.82 | <0.30 | ||
| SMA/1999–2000b | AM | 1.7 | 2.5 | 0.87 | 7.1 | 7.7 | |
| 14 | GM | 0.87 | 2.0 | 0.54 | 5.9 | 4.6 | |
| Min–max | <0.01–8.3 | 0.51–8.0 | 0.07–3.5 | 2.0–18 | 0.62–32 | ||
| Recycling of SMA/1999–2000b | AM | 0.50 | 5.4 | 6.3 | 21 | 10 | |
| 8 | GM | 0.42 | 2.2 | 2.1 | 8.8 | 5.1 | |
| Min–max | 0.13–0.82 | 0.50–22 | 0.29–24 | 1.8–78 | 1.2–29 | ||
Urinary PAH metabolites
Workers’ exposures were monitored by measuring the concentration of naphthols, phenanthrols and 1-OHP in pre- and post-shift urine as shown in Fig. 1. No significant differences could be observed between these biomarker excretion levels either in non-smoking or in smoking men working at four different sites and in crews laying asphalt mixes of the AC, AC-WPT, SMA, or SMA-WPT. The greatest differences displayed in urinary OH-PAH concentrations were associated with tobacco smoking. Particularly, the levels of urinary 1- and 2-naphthol and 1-OHP were higher in smokers than in non-smokers, but no such clear difference in the excretion of urinary phenanthrols could be observed. The concentrations of OH-PAH metabolites were low in all pavers, except for one subject (roller driver) in the AC group. The 1-OHP level of this subject both before and after his shift was as high as 2.65 and 2.0 μmol mol−1 creatinine, respectively. This high 1-OHP excretion level was apparently mainly attributable to the high cumulative uptake of pyrene/PAH already before the start of the workday. The OH-PAHs assayed in the urine of two traffic controllers (smokers) showed a clearly lower excretion level of phenanthrols than the concentrations observed in road pavers, while the naphthol and 1-OHP levels were comparable to those detected in road pavers. The data summarized in Table 6 indicate that no marked differences could be observed in the urinary excretion of 1- and 2-naphthol, 1-, 2-, 3-, 4- and 9-phenanthrols, or 1-OHP between the pavers exposed to bitumen fumes of conventional asphalt (AC or SMA) or asphalt mixes containing waste plastic and tall oil pitch. The pre- and post-shift urine concentrations of naphthols, phenanthrols and 1-OHP were 3.0–3.7-fold, 1.2–1.6-fold and 1.6–3.5-fold, respectively, lower in the non-smokers than in the smokers (Table 6).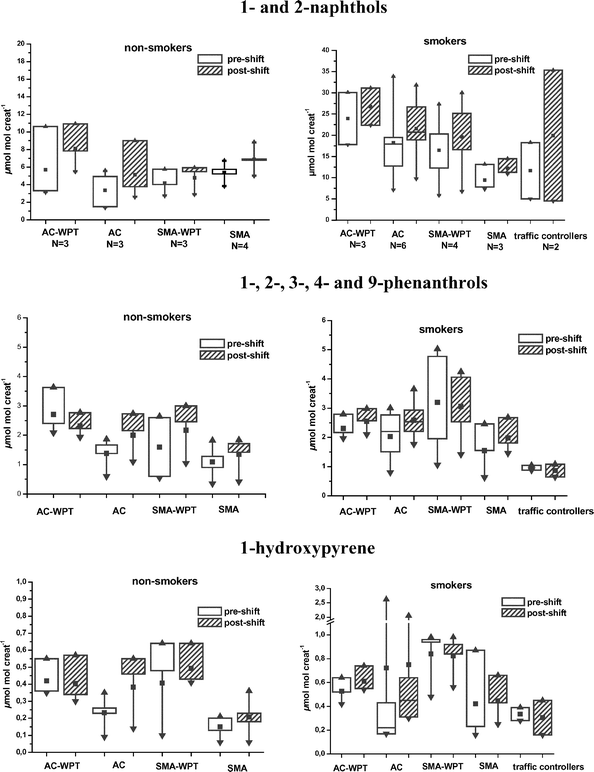 | ||
| Fig. 1 OH-PAH metabolites in urine (μmol mol−1 creatinine) of asphalt pavers laying asphalt with and without waste material. AC-WPT = asphalt concrete containing waste plastics and tall oil pitch as a binder, AC = asphalt concrete, SMA-WPT = stone mastic asphalt containing waste plastics and tall oil pitch as a binder, SMA = stone mastic asphalt, ▲ = maximum concentration, ■ = arithmetic mean, ▼ = minimum concentration, — = median, 50% of the values fall within the box. | ||
| Measurement | Conventional asphaltb | WPT asphaltb | ||||||
|---|---|---|---|---|---|---|---|---|
| Preshift | Postshift | Preshift | Postshift | |||||
| AM ± SD | GM (range) | AM ± SD | GM (range) | AM ± SD | GM (range) | AM ± SD | GM (range) | |
| a Values are the AM, arithmetic mean ± SD, GM, geometric mean (range). b Asphalt mixture types: concrete asphalt and stone mastic asphalt. Differs significantly from the corresponding value in non-smokers (Student’s t-test): *p < 0.05. Differs significantly from the corresponding value in the waste plastic–tall oil pitch asphalt exposed group (Student’s t-test): § p < 0.05. | ||||||||
| Smokers (n = 8) | Smokers (n = 6) | |||||||
| 1- and 2-Naphthol | 14.9 ± 9.0* | 13.0 (7.1–33.9) | 18.1 ± 8.0* | 16.6 (9.7–31.8) | 18.9 ± 9.1* | 16.6 (5.8–30.1) | 22.0 ± 9.2* | 19.7 (6.8–31.1) |
| 1-Naphthol | 6.1 ± 4.2* | 4.8 (1.9–14.7) | 7.4 ± 4.2* | 6.4 (3.0–14.1) | 8.3 ± 4.7* | 6.9 (1.9–15.1) | 9.4 ± 4.7* | 7.6 (1.5–13.7) |
| 2-Naphthol | 8.9 ± 5.0* | 7.9 (4.8–19.2) | 10.6 ± 4.3* | 10.0 (6.1–19.1) | 10.6 ± 4.5* | 9.6 (3.9–15.5) | 12.6 ± 4.5* | 11.8 (5.2–17.4) |
| Phenanthrols | ||||||||
| 1-,2-,3-,4-,9-OHPh | 1.91 ± 0.87* | 1.69 (0.63–3.00) | 2.41 ± 0.71* | 2.31 (1.46–3.65) | 2.82 ± 1.65 | 2.44 (1.06–5.02) | 2.82 ± 1.11 | 2.63 (1.42–4.24) |
| 1-OHPh | 0.85 ± 0.55 | 0.66 (0.15–1.78) | 0.88 ± 0.45 | 0.77 (0.31–1.66) | 1.54 ± 1.14 | 1.21 (0.39–3.12) | 1.48 ± 0.91 | 1.26 (0.53–2.74) |
| 2-,3-OHPh | 0.59 ± 0.24* | 0.55 (0.31–0.91) | 0.96 ± 0.18* | 0.94 (0.72–1.20) | 0.82 ± 0.50 | 0.71 (0.43–1.73) | 0.85 ± 0.21 | 0.83 (0.59–1.11) |
| 4-,9-OHPh | 0.47 ± 0.20* | 0.41 (0.12–0.67) | 0.57 ± 0.21* | 0.53 (0.29–0.81) | 0.46 ± 0.18 | 0.43 (0.21–0.65) | 0.49 ± 0.14 | 0.47 (0.30–0.64) |
| 1-Hydroxypyrene | 0.61 ± 0.84* | 0.36 (0.16–2.62) | 0.66 ± 0.58* | 0.53 (0.25–2.05) | 0.72 ± 0.27* | 0.67 (0.42–0.98) | 0.76 ± 0.18* | 0.75 (0.54–0.98) |
| Total sum | 17.4 ± 9.6* | 15.5 (8.6–36.5) | 21.1 ± 8.5* | 19.7 (13.0–35.0) | 22.5 ± 9.0* | 20.9 (11.8–33.0) | 25.6 ± 9.0* | 24.1 (11.9–34.9) |
| Non-smokers (n = 8) | Non-smokers (n = 7) | |||||||
| 1- and 2-Naphthol | 4.6 ± 1.7 | 4.2 (1.4–6.7) | 6.0 ± 2.3 | 9.0 (2.6–9.0) | 5.1 ± 2.7 | 4.6 (2.7–10.6) | 6.8 ± 2.6 | 6.3 (2.9–10.9) |
| 1-Naphthol | 1.7 ± 1.0 | 1.3 (0.1–3.2) | 1.9 ± 1.3 | 1.3 (0.1–3.4) | 1.8 ± 0.9 | 1.6 (0.6–3.2) | 2.4 ± 1.2 | 2.1 (1.2–4.8) |
| 2-Naphthol | 2.9 ± 1.0 | 2.7 (1.3–4.3) | 4.1 ± 1.4 | 3.9 (2.0–5.9) | 3.3 ± 2.3 | 2.8 (1.3–8.3) | 4.4 ± 2.0 | 3.9 (1.4–6.7) |
| Phenanthrols | ||||||||
| 1-,2-,3-,4-,9-OHPh | 1.21 ± 0.55§ | 1.07 (0.35–1.86) | 1.70 ± 0.72 | 1.51 (0.42–2.73) | 2.39 ± 0.94 | 2.12 (0.55–3.63) | 2.35 ± 0.69 | 2.23 (1.05–3.00) |
| 1-OHPh | 0.64 ± 0.31 | 0.55 (0.16–1.01) | 0.71 ± 0.33 | 0.61 (0.17–1.07) | 1.28 ± 0.52 | 1.11 (0.24–1.77) | 1.09 ± 0.51 | 0.96 (0.29–1.95) |
| 2-,3-OHPh | 0.32 ± 0.15 | 0.29 (0.11–0.57) | 0.64 ± 0.29 | 0.57 (0.15–1.20) | 0.51 ± 0.15 | 0.47 (0.18–63) | 0.73 ± 0.20 | 0.70 (0.48–1.04) |
| 4-,9-OHPh | 0.25 ± 0.14 | 0.22 (0.09–0.51) | 0.35 ± 0.16 | 0.31 (0.09–0.56) | 0.60 ± 0.57 | 0.45 (0.13–1.85) | 0.53 ± 0.16 | 0.51 (0.28–0.73) |
| 1-Hydroxypyrene | 0.17 ± 0.07§ | 0.15 (0.06–0.26) | 0.27 ± 0.15§ | 0.22 (0.06–0.55) | 0.45 ± 0.19 | 0.39 (0.10–0.64) | 0.46 ± 0.13 | 0.45 (0.30–0.64) |
| Total sum | 6.0 ± 2.1 | 5.6 (2.1–8.8) | 8.0 ± 2.8 | 7.6 (3.8–12.3) | 7.9 ± 2.8 | 7.5 (4.7–13.1) | 9.6 ± 3.0 | 9.2 (5.8–14.3) |
Questionnaire
Most paving workers (12/14) experienced the odour of WPT asphalt as being more unpleasant than that of conventional asphalt. Two of them reported also headache and nausea. WPT asphalt workers had eye irritation (12/14), hoarseness of throat (6/14) and redness of eyes (5/14). During the laying of SMA-WPT, the pavers reported also itching and irritation of skin. Only four pavers out of 15 reported eye irritation and one complained of throat irritation during laying of conventional asphalt. The concentration of aldehydes explained eye (p = 0.01) and skin (p = 0.04) irritation and hoarseness of throat (p = 0.01). Bitumen fumes explained also hoarseness of throat (p = 0.01) and resin acids explained eye irritation (p = 0.02).Most of the paving workers (20/29) wore gloves, but only a few workers protected their legs and arms with clothes. Some of the paving workers (9/29) used light fuel oil for cleaning their tools during paving. The use of gloves and light fuel oil did not affect statistically significantly on the level of dermal contamination with unalkylated PAHs or urinary metabolite post-shift concentrations of phenanthrols and 1-OHP. However, the use of light fuel oil exhibited an association with the concentration of urinary naphthols in the post-shift samples (p = 0.03).
Discussion
Modifying of AC and SMA asphalts with waste plastic and tall oil pitch (AC-WPT and SMA-WPT) did not increase significantly the concentration of total particulates, bitumen fumes, bitumen vapour or PAHs in the breathing zone of the workers at the paving sites. In contrast to our results, it was reported that the use of crumb rubber in asphalt mixtures increased the level of total particulates and bitumen fumes by two-fold when compared to the conventional asphalt laid at the same laying temperatures.2 In another study, the use of WPT asphalt increased also total particulates, bitumen fumes and PAHs in air.15 In that study, the waste plastic was composed of polyethylene and polypropylene, polystyrene, polyvinyl chloride and polymethyl methacrylate and the laying temperatures were stated to be at the same level or 20 °C higher than in our study.The maximum bitumen fume concentration, 0.29 mg m−3, was measured at the breathing zone of the screed man during laying of AC-WPT. The concentrations of bitumen fumes were thus below 0.5 mg m−3, the threshold limit value (TLV) recommended by the American Conference of Governmental Industrial Hygienists (ACGIH).33 The level of air impurities measured at the SMA and SMA-WPT paving sites was only one quarter of that reported previously for SMA paving, probably due to the lower laying temperatures applied (Table 3).8 At the AC and AC-WPT sites, the air impurities were at the same level as previously noted during AC paving (Table 3).3,34 According to the asphalt workers exposure database (AWE Database) that includes data from several European countries from the period 1970–2000, the geometric mean concentration of bitumen fume was at the same level as that found in this study.16,35
The use of waste plastics and tall oil pitch in asphalt increased the emissions of aldehydes (p < 0.0001) and resin acids. The sum concentration of 11 aldehydes at the laying of AC-WPT and SMA-WPT was 14 and 4 times that measured at the laying of AC and SMA, respectively. However, the concentrations of measured aldehydes were low when compared to the current TLVs (ACGIH).33 At the WPT asphalt sites, the concentration of formaldehyde was approx. 10% of its TLV. Formaldehyde is classified by IARC as carcinogenic to humans [class 1]36 and acetaldehyde as possibly carcinogenic to humans [class 2B].37 Aldehydes are not components in bitumen, but they can be formed in the heating of organic materials. In contrast to our results, aldehydes were not found in conventional asphalt paving in Sweden.38 When asphalts have contained organic additives such as styrene-butadiene-styrene polymer or tall oil pitch, the aldehydes have been detected at the same level as in this study.34 The most abundant aldehydes measured in the emissions of asphalt and WPT asphalt were aliphatic aldehydes and benzaldehyde and their alkylated compounds, which are not such strong irritants as unsaturated aliphatic aldehydes, e.g., crotonaldehyde or acrolein.39
Various uses of rosin and exposure to its resin acid constituents have been associated with dermal and pulmonary sensitization.40 Dermal sensitization and the occupational asthma suffered by solderers exposed to thermal decomposition products in gum rosin core solder have been associated with the abietic acids40 and with their oxidised derivatives (peroxides).41 We detected resin acids in air samples during laying of WPT asphalt. This indicates that the mixing of tall oil pitch into asphalt may pose a health risk. Tall oil and its pitch contain abietic acids such as found in gum rosin.18 The highest level of resin acids, 42 μg m−3, measured at the rear of the paving machine, is comparable with the British OEL value for resin acids in soldering fumes.42 There are several reasons why it is difficult to predict the risk of occupational asthma in road pavers. The sensitizing capacity of different rosins may differ. The potency of Finnish and Swedish tall-oil rosins to induce skin sensitization has been weak in comparison with products from other parts of Europe.43 Another study proposed that tall-oil rosin may be less potent as a contact allergen than gum rosin.44 The abietic acid fraction in gum rosins may differ from that in tall oil pitch. Conceivably, if oxidised derivatives of resin acids are the allergy causing agents in rosin fumes, the formation of these peroxides will probably depend on the heating temperature that is lower in paving (Table 1) than in soldering (200–350 °C). As a precaution for minimizing the sensitization risk, our recommendations are the same as ACGIH has issued for rosin core soldering: exposure to resin acids during heating of tall oil pitch products should be maintained as low as possible.
The dermal PAH exposure was low being slightly lower on the skin of the WPT asphalt workers than on the conventional asphalt workers. This difference was seen also in air samples; one explanation might be the lower bitumen content in WPT asphalt than in conventional asphalt, i.e. some of the bitumen had been replaced with waste plastic and tall oil pitch in the WPT asphalt. The concentration of total PAHs on the workers’ skin pads was only 16–24% of the dermal PAH exposure in SMA (GM 5.9 ng cm−2) and remixing paving workers (GM 8.8 ng cm−2) measured in our previous study.9 The comparison of dermal PAH exposure results is difficult due to different analysing and sampling methods for substances in bitumen fumes (unalkylated PAHs, alkylated PAHs and sulfur-containing aromatic compounds).45 McClean et al. have measured significant deposition of pyrene (GM 3.5 ng cm−2) and polycyclic aromatic compounds, both unalkylated and alkylated PAHs (GM 89 ng cm−2) on the dermal patches worn by asphalt workers.6 In our study, the deposition of pyrene was only 0.1 ng cm−2 (GM). The dermal PAH exposure of the asphalt workers has been in the range of 1–20 ng cm−2 and the GM of pyrene on the skin has been 0.5–3.5 ng cm−2.6,9–11
The paving temperature is a significant determinant of asphalt induced airborne emissions. An increase in the temperature of the asphalt mixes increases both the amount of emissions38,46 and the proportion of particulate bound PAH compounds8,25,47 and probably also the amounts of decomposition products, such as aldehydes, resin acids and their peroxides, in the emissions. Our results support the proposal that keeping the asphalt temperature as low as possible is a way to reduce exposure.3,25,48 Low asphalt temperature decreases probably also the deposition of bitumen fumes on the skin. In this study, most of the paving workers (20/29) used gloves, but only a few wore clothes for protecting their legs and arms. The skin contamination and the urinary phenanthrols and 1-OHP were at the same level in those workers using fuel oil for cleaning of tools as in those not using fuel oil. The amount of light fuel oil used was minuscule and the workers were careful to avoid splashing and skin staining. However, excessive and careless use of light fuel oil would devote the dermal PAH exposure and also the amount of the PAH-metabolites in urine.9,49
The paving workers described the emissions from asphalts containing waste plastic and tall oil pitch as being worse eye, skin and respiratory irritating agents than emissions from conventional asphalt. When statistically tested, aldehydes explained eye, skin and respiratory irritation, resin acids also explained eye irritation.
The concentrations of naphthols, phenanthrols and 1-OHP in post shift urine of the asphalt workers were generally low and varied within the range levels: 2.6–31.8, 0.42–4.24 and 0.06–2.05 μmol mol−1 creatinine, respectively. As expected, also the exposure levels to the parent PAHs, naphthalene, phenanthrene and pyrene were relatively low as revealed by ambient and dermal exposure measurements (Table 3 and 5). Exposure of workers to carcinogenic PAHs was low, e.g., the concentrations of BaP were below the quantification limit (0.01 μg m−3). According to the results of the present PAH exposure assessments, there was no significant difference between pavers laying conventional asphalt or WPT asphalt. On the other hand, there was a highly significant difference between non-smoking and smoking workers. Despite the similar PAH exposure data (Table 3 and 5), the smokers had consistently higher OH-PAH levels in urine than the non-smokers. Smoking increased to a lesser extent the urinary levels of phenanthrols compared to the smoking induced elevations in excretion of naphthols or 1-OHP. Ambient, dermal and biological exposure assessment data from our previous surveys in 1990–2003 suggest that paving-related exposure to PAH carcinogens has been in general relatively low among Finnish asphalt workers.3,8,9 Addition of industrial by-products in asphalt mixes, such as coal fly ash8,9 or waste plastics and tall oil pitch, could not be demonstrated to increase workers’ exposure to PAHs. Instead it seems that high laying temperatures and asphalt recycling processes are factors that can enhance the risk of exposure to PAHs.
Conclusions
In comparison to the impurities emitted from conventional asphalt mixes (SMA, AC), the use of waste plastic and tall oil pitch as a binder in asphalt resulted in a many-fold higher concentrations of diverse aldehydes and in quantifiable levels of emission of resin acids (≤42 μg m−3). The sensitizing effect by resin acids may pose a greater health threat than that attributable to the aldehydes, which are primarily irritating compounds. Moreover, the WPT asphalt workers complained of more irritation in eyes, throat and skin compared to the pavers exposed to conventional asphalt. The workers’ exposure to total particulates, bitumen fumes and vapours and PAH was similar and low at all paving sites. The available data show that unlike inorganic additives, the presence of organic materials in asphalt mixes may increase the concentration of air impurities which can be hazardous to health.Abbreviations
Waste plastics (WP), tall oil pitch (T), stone mastic asphalt (SMA), asphalt concrete (AC), asphalt containing waste material (SMA-WPT and AC-WPT), polycyclic aromatic hydrocarbons (PAH), benzo(a)pyrene (BaP), 1-hydroxypyrene (1-OHP), monohydroxylated PAHs (OH-PAH).Acknowledgements
We wish to thank the Emil Aaltonen Foundation, the Finnish Work Environment Fund, the Finnish National Road Administration and Arizona Chemical for financial support and the workers for participating in this study. Ms Beatrice Bäck, Mr Tom Johnsson and Ms Maj-Len Henriks-Eckerman are thanked for air impurity analysis and Mr Jouni Mikkola for the urine analyses of hydroxy PAHs.References
- I. Burstyn, B. Randem, J. E. Lien, S. Langard and H. Kromhout, Ann. Occup. Hyg., 2002, 46(1), 79–87 CrossRef CAS.
- G. Burr, A. Tepper, A. Feng, L. Olsen and A. Miller, NIOSH HEALTH HAZARD EVALUATION REPORT: HETA 2001-0536-2864 Crumb-Rubber Modified Asphalt Paving: Occupational Exposures and Acute Health Effects, National Institute for Occupational Safety and Health, Cincinnati, Ohio, 2001, p. 42 Search PubMed.
- P. Heikkilä, R. Riala, M. Hämeilä, E. Nykyri and P. Pfäffli, AIHA J., 2002, 63, 156–165 Search PubMed.
- J. O. Levin and B. Järvholm, Am. J. Ind. Med., 1999, 36(suppl. 1), 147–148 CrossRef.
- B. Järvholm, G. Nordström, B. Högstedt, J. O. Levin, J. Wahlström, C. Östman and C. Bergendahl, Scand. J. Work Environ. Health, 1999, 25(2), 131–136 Search PubMed.
- M. D. McClean, R. D. Rinehart, L. Ngo, E. A. Eisen, K. T. Kelsey and R. F. Herrick, Ann. Occup. Hyg., 2004, 48(8), 663–671 CrossRef CAS.
- M. D. McClean, R. D. Rinehart, L. Ngo, E. A. Eisen, K. T. Kelsey, J. K. Wiencke and R. F. Herrick, Ann. Occup. Hyg., 2004, 48(6), 565–578 CrossRef CAS.
- V. Väänänen, M. Hämeilä, H. Kontsas, K. Peltonen and P. Heikkilä, J. Environ. Monit., 2003, 5, 739–746 RSC.
- V. Väänänen, M. Hämeilä, E. Nykyri, P. Kalliokoski and P. Heikkilä, Ann. Occup. Hyg., 2005, 49(2), 167–178 CrossRef CAS.
- Q. Zhou, PhD thesis, University of Cincinnati, 1997.
- J. B. Hicks, Appl. Occup. Environ. Hyg., 1995, 10(10), 840–848 CAS.
- J. Lien, Asphaltprojektet 1991–92, Forelöpig rapport etter målinger hösten 1991, Statens vegvesen, Oslo, 1992 Search PubMed.
- ASTM, Use of Waste Materials in Hot-Mix Asphalt, ed. H. F. Waller, ASTM, Philadelphia, PA, vol. 1193, 1993, p. 306 Search PubMed.
- V. Väänänen, M. Hämeilä, P. Peltonen, A. Saarela and P. Heikkilä, Asfaltissa käytettävien kierrätysmateriaalien aiheuttamat työterveyshaitat, Finnish Institute of Occupational Health (FIOH), Helsinki, 2003, p. 36 Search PubMed.
- J. Paananen, MSc thesis, University of Jyväskylä, 1998.
- I. Burstyn, H. Kromhout, T. Kauppinen, P. Heikkilä and P. Boffetta, Ann. Occup. Hyg., 2000, 44(1), 43–56 CAS.
- P. Heikkilä, V. Väänänen, M. Hämeilä and K. Linnainmaa, Toxicol. Vitro, 2003, 17, 403–412 Search PubMed.
- B. Holmbom and V. Erä, J. Am. Oil Chem. Soc., 1978, 55(3), 342–344 CrossRef CAS.
- IARC, Bitumens, Coal-tars and Derived Products, Shale-oils and Soots, in IARC Monographs on the Evaluation of The Carcinogenic Risk of Chemicals to Humans; Polynuclear Aromatic Compounds, Part 4, International Agency for Research on Cancer (IARC), Lyon, 1985 Search PubMed.
- IARC, Bitumens and extracts of steam-refined and air-refined bitumens., in IARC monographs on the evaluation of carcinogenic risks to humans, suppl. 7, International Agency for Research on Cancer, Lyon, 1987 Search PubMed.
- S. Binet, A. Pfohl-Leszkowicz, H. Brandt, M. Lafontaine and M. Castegnaro, Sci. Total Environ., 2002, 300(1–3), 37–49 CrossRef CAS.
- WHO, Asphalt (Bitumen), in Concise International Chemical Assessment Document 59, 2004, World Health Organization, Geneva Search PubMed.
- P. Boffetta, I. Burstyn, T. Partanen, H. Kromhout, O. Svane, S. Langard, B. Jarvholm, R. Frentzel-Beyme, T. Kauppinen, I. Stucker, J. Shaham, D. Heederik, W. Ahrens, I. A. Bergdahl, S. Cenee, G. Ferro, P. Heikkila, M. Hooiveld, C. Johansen, B. G. Randem and W. Schill, Am. J. Ind. Med., 2003, 43(1), 18–27 CrossRef.
- P. Boffetta, I. Burstyn, T. Partanen, H. Kromhout, O. Svane, S. Langard, B. Jarvholm, R. Frentzel-Beyme, T. Kauppinen, I. Stucker, J. Shaham, D. Heederik, W. Ahrens, I. A. Bergdahl, S. Cenee, G. Ferro, P. Heikkila, M. Hooiveld, C. Johansen, B. G. Randem and W. Schill, Am. J. Ind. Med., 2003, 43(1), 28–39 CrossRef.
- T. Norseth, J. Waage and I. Dale, Am. J. Ind. Med., 1991, 20(6), 737–744 CrossRef CAS.
- B. G. Randem, B. Ulvestad, I. Burstyn and J. Kongerud, Occup. Environ. Med., 2004, 61(4), 367–369 CrossRef CAS.
- T. Vu-Duc, C. K. Huynh and P. Boiteux, Mikrochim. Acta, 1995, 120, 271–280.
- J. J. Sauvain, T. Vu-Duc and C. K. Huynh, Fresenius’ J. Anal. Chem., 2001, 371(7), 966–974 CrossRef CAS.
- US EPA, EPA-T0-11A. Compendium of methods for the determination of toxic organic compounds in ambient air, 1999, US Environmental Protection Agency, Cincinnati Search PubMed.
- L. D. Metcalfe and C. N. Wang, J. Chromatogr. Sci., 1981, 19, 530–535 CAS.
- E. Elovaara, V. Väänänen and J. Mikkola, Arch. Toxicol., 2003, 77, 183–193 CAS.
- E. Elovaara, J. Mikkola, M. Mäkelä, B. Paldanius and E. Priha, Toxicol. Lett., 2005 DOI:10.1016/j.toxlet.2005.09.028.
- ACGIH, 2005 TLVs and BEIs; Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices, 2005, ACGIH (American Conference of Governmental Industrial Hygienists), Cincinnati, Ohio Search PubMed.
- P. Heikkilä, R. Riala, M. Hämeilä and P. Pfäffli, Bitumihuurut - käytetyt aineet ja altistuminen tienpäällystys- ja vedeneristystöissä, Finnish Institute of Occupational Health (FIOH), Helsinki, 1994, p. 57 Search PubMed.
- I. Burstyn and H. Kromhout, Risk Anal., 2000, 20(5), 653–663 Search PubMed.
- IARC, Volume 88. Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxy-2-propanol, International Agency for Research on Cancer (IARC), Lyon, 2004 Search PubMed.
- IARC, Volume 71. Re-Evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide, International Agency for Research on Cancer (IARC), Lyon, 1999 Search PubMed.
- L. G. Ekström, Bitumen fumes and exposures to solvents in connection with the laying of asphalt; Report from exposure measurements carried out in 1990, Nynäs Bitumen Technology AB, Johanneshov, Sweden, 1991 Search PubMed.
- W. H. Steinhagen and C. S. Barrow, Toxicol. Appl. Pharmacol., 1984, 72(3), 495–503 CrossRef CAS.
- ACGIH, Documentation of the Threshold Limit Values and Biological Exposure Indices, ACGIH (American Conference of Governmental Industrial Hygienists), 7th edn, 2001 Search PubMed.
- P. A. Smith, D. R. Gardner, D. B. Drown, W. W. Jederberg and K. Still, Am. Ind. Hyg. Assoc. J., 1998, 59(12), 889–894 CrossRef CAS.
- HSE, Health and Safety Executive EH 40/2002 Occupational Exposure Limits 2002, Norwich, 2002 Search PubMed.
- B. Hausen and C. Hessling, Contact Dermatitis, 1990, 23(2), 90–95 CAS.
- G. Farm, C. Liden and A. Karlberg, Contact Dermatitis, 1994, 31(2), 102–107 CAS.
- S. Binet, A. Pfohl-Leszkowicz, H. Brandt, M. Lafontaine and M. Castegnaro, Sci. Total Environ., 2002, 300, 37–49 CrossRef CAS.
- Concawe, Assessment of personal inhalation exposure to bitumen fume; guidance for monitoring benzene-soluble inhalable particulate matter, in Concawe report no. 7/02, Concawe, Brussels, 2002, p. 31 Search PubMed.
- A. Kitto, M. Pirbazari, B. Badriyha, V. Ravindran, R. Tyner and C. Synolakis, Environ. Technol., 1997, 18, 121–138 CrossRef CAS.
- H. C. Brandt and P. C. de Groot, Am. Ind. Hyg. Assoc. J., 1999, 60(2), 182–190 CrossRef CAS.
- R. A. Weker, R. F. Herrick and R. D. Rinehart, J. Occup. Environ. Hyg., 2004, 1(5), 334–342 Search PubMed.
- V. Väänänen, MSc Lic thesis, University of Kuopio, 2005.
Footnote |
| † Presented at the Fifth International Symposium on Modern Principles of Air Monitoring & Biomonitoring, June 12–16 2005, Norway. |
| This journal is © The Royal Society of Chemistry 2006 |
