Chemistry at high pressure
Paul F. McMillanab
aDepartment of Chemistry and Materials Chemistry Centre, University College London, Christopher Ingold Laboratory, 20 Gordon Street, London, WC1 H0AJ, United Kingdom. E-mail: p.f.mcmillan@ucl.ac.uk.; Fax: 020 7679 7463; Tel: 020 7679 4610
bDavy–Faraday Research Laboratory, Royal Institution, 21 Albemarle Street, London, W1X 4BS, United Kingdom
 Paul F. McMillan | Paul F. McMillan is Professor of Chemistry at University College London and in the Davy–Faraday laboratory at the Royal Institution. He is also Director of the UCL Materials Chemistry Centre. He graduated with a BSc in Chemistry from Edinburgh University in 1977 and moved to Arizona State University for his PhD (1981). He became Professor there in 1992 and Director of the Centre for Solid State Science (1997). Since moving to London in 2000, he has established laboratories for developing solid state chemistry and new materials research under high pressure conditions, extending to biology and nanomaterials. He was awarded a Wolfson–Royal Society Research Merit Award (2001–2006) and an EPSRC Senior Research Fellowship (2006–2011). He was elected Fellow of the RSC and received the award for Solid State Chemistry (2003). He has been awarded an EPSRC Senior Research Fellowship (2006) to develop the field of high pressure chemistry. |
Chemistry involves the study of the reactions and properties of the elements and their compounds. After over two centuries of systematic chemical research, combined with the modern use of spectroscopy and diffraction methods to determine structure and valence states and detailed quantum mechanical investigations of interatomic bonding; it is now generally accepted that the fundamentals of chemistry are understood and established. New frontiers that are being developed now lie in the supramolecular chemistry of biological molecules, or in studying the synthesis and properties of polymeric macromolecules and nanoparticles. However, we must remember that most of our chemical knowledge has been gained from studies carried out at or near one atmosphere pressure at the Earth's surface, while much of the matter in the Universe exists under much higher pressure conditions, deep inside planets and stars.
Chemistry is concerned with the behaviour of the outermost electrons of atoms that determine the bonding, reactivity and structures of molecules and solids. By the time a typical solid or liquid is compressed to above a few hundred thousand atmospheres, its molar volume is reduced by approximately 50%. Once the megabar range (1,000,000 atm; P = 100 GPa; 1 Mbar = 1,000 kbar) is reached, average interatomic distances can be decreased by up to a factor of two. It is to be expected that large changes will occur in the outer electron shells under extreme densification conditions, and that these will lead to substantial modifications of the chemical and physical properties. It is known that such large changes in molecular and electronic structure do in fact occur, and that the very arrangement of the Periodic Table might have to be modified for high pressure conditions.
As a simple example, we can consider the typical alkaline earth metals such as Ca and Sr that possess a fully close-packed fcc structure at ambient conditions. However, pressurising Ca to P > 200 kbar (20 GPa) causes it to transform to a less efficiently packed bcc structure, with a lower coordination of the metal atoms1 (Fig. 1). A similar transition occurs for Sr at lower pressure (3.5 GPa). This transformation is due to pressure-induced mixing occurring between 3d and 4s electronic shells, giving Ca and Sr the character of transition metals rather than alkaline earth elements at high pressure. Likewise, K begins to form compounds and solid-state solutions with Ag and Ni above P > 10 GPa, indicating a transition metal-like character for this element also.2 Such changes in the chemical reactivity and molecular or solid state packing in highly densified elements and their compounds can be linked to expected changes in preferred oxidation states, along with the appearance of unusual valencies and bonding patterns, in high pressure chemistry.3–7 In this special issue of Chemical Society Reviews, we present topics of current interest in high pressure research that are relevant to chemistry. The present issue is particularly timely, in that it appears just over one hundred years after P. W. Bridgman began his pioneering experiments that revolutionised high pressure research.8–10
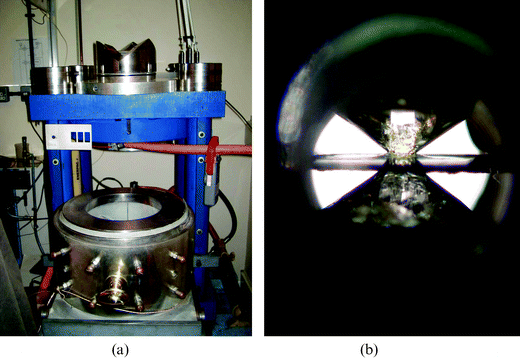 | ||
| Fig. 1 High pressure devices used to conduct high-pressure chemistry experiments. (a) A multi-anvil “large volume” device installed in the basement Davy–Faraday laboratory and Materials Chemistry Centre at UCL. It enables high-pressure, high-temperature syntheses and in situ experiments (electrical conductivity, differential thermal analysis) on solid–liquid phase samples at up to approximately 25 GPa and 1800–2000 °C. It is most useful for carrying out synthesis-quench experiments to recover new compounds and materials for laboratory study and potential technology development. (b) A side view of the diamond anvil cell, that enables in situ studies via optical spectroscopy and X-ray diffraction (usually carried out at a synchrotron facility) up to and beyond the megabar range (P ∼ 100 GPa). The sample is mounted between the flat culet faces (e.g., 30–500 µm) of two gem-quality diamonds and compressed by applying mechanical force to the large outer table. The experimental chamber is defined by a hole drilled in a metal or ceramic gasket that surrounds the sample. | ||
Modern high pressure experiments are enabled by various types of high-pressure device, ranging among the large mechanical presses developed by Bridgman and subsequent generations of high-pressure researchers, through the hand-held diamond anvil cells (DACs) that now enable physics and chemistry experiments to be carried out in the laboratory into the multimegabar range (P > 100 GPa), to specially-designed windowed pressure cells for chemistry and biology experiments in the low-pressure range (Fig. 1).1,11 The most extreme P–T conditions currently available are developed in dynamic shock-wave environments, that allow sampling of deep planetary interiors including the gas giants and small stars, and also conditions experienced during thermonuclear explosions.1,11–13 Bridgman's experimental strategies were quickly adopted and developed by geoscientists seeking to understand the deep interior of the Earth and other planets,1,13 and also by solid-state researchers aiming to synthesise ultra-hard materials like diamond and cubic BN.9,14 In this issue, E. Horvath-Bordon and co-workers describe modern high-pressure research into the synthesis of new technologically-relevant nitride materials, combining experiments with theoretical calculations.
Ultimately, it. is thought that most substances should become metallic at the most extreme pressures, as the close approach of atoms results in electronic overlap.15 It is generally found that the electronic bandgaps of insulators and semiconductors (and also the LUMO–HOMO gap within molecular compounds) decrease with increasing pressure, with a few notable exceptions that include dense MgO and diamond.4,9,13,16 Elements such as iodine become monatomic metals by around 20 GPa (P = 200 kbar). The well-known diamond-structured semiconductor Si has transformed into metallic polymorphs by 10–12 GPa.1 Once megabar pressures are attained (i.e., P ∼ 100 GPa), elements such as oxygen, sulfur and xenon along with typical “ionic” solids like CsI have all become metals, and many of these are superconductors.17–19 In fact, the Periodic Table of superconductivity among the elements is almost complete by this pressure range.19 These results constitute a series of remarkable achievements for high-pressure physics. However, changes in the electronic structure that are even more interesting and relevant for chemistry are observed to occur throughout the 1–100 GPa pressure range, where asymmetries in the electron density distribution appear that determine the physical and chemical properties of elements and their compounds.
One of the primary targets of high pressure research over several decades has been to achieve the long-predicted metallisation of the first element, hydrogen, as a monatomic solid15,20–22 Aside from its fundamental significance for atomic and molecular theory, this result is needed to explain astrophysical observations such as the magnetic field of Jupiter. However, despite the apparent electronic simplicity of element 1H, its behaviour under extreme densification is found to be remarkably complex. Metallic conductivity has now been reported in the high temperature fluid state from dynamic shock-wave experiments.12 However, formation of solid metallic hydrogen has proved elusive, despite studies carried out to over 3 megabars.20,21 Instead, the electron density within the H2 molecule becomes asymmetric, resulting in Hδ+–Hδ− species with strong infrared activity.22 Here there are implications for developing new crystal structures and reaction chemistry among polar species of H2 at high pressure. Similar asymmetries in the electron density appear for NO2 and N2O, that form molecular ionic solids like (NO+ NO3−) at high pressure.23,24 On the other hand, the hydrogen bonding in H2O becomes more symmetric at high pressure.25 The high-pressure chemistry of H-containing solids is reviewed by A. Goncharov and R. J. Hemley in this issue. CO2 is well known to form strongly-bound molecular species under low pressure conditions. However, following high T treatment at high P, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bonds are replaced by singly-bonded polymerised CO44− groups that form crystalline and amorphous framework structures analogous to SiO2 and GeO2.26,27 The solid-state polymorphism of CO2 under high-P,T conditions is reviewed here by M. Santoro and F. Gorelli. In the elemental solid state, “simple” metals or metalloids such as Rb, Sr, Ba, Te, Se and Ga exhibit unusual charge disproportionation reactions at high pressure to result in distinct sublattices in which the atoms have different formal oxidation states, that are often incommensurate with one another to result in very large unit cells. The crystallography of the new “elemental alloys” is reviewed here by M. McMahon and R. J. Nelmes.
O double bonds are replaced by singly-bonded polymerised CO44− groups that form crystalline and amorphous framework structures analogous to SiO2 and GeO2.26,27 The solid-state polymorphism of CO2 under high-P,T conditions is reviewed here by M. Santoro and F. Gorelli. In the elemental solid state, “simple” metals or metalloids such as Rb, Sr, Ba, Te, Se and Ga exhibit unusual charge disproportionation reactions at high pressure to result in distinct sublattices in which the atoms have different formal oxidation states, that are often incommensurate with one another to result in very large unit cells. The crystallography of the new “elemental alloys” is reviewed here by M. McMahon and R. J. Nelmes.
Although he received the Physics Nobel prize (1946) for his work, Bridgman's research had an enormous impact on various areas of high pressure chemistry.8,28 High pressure not only causes modification of the electronic structures and the crystal packing resulting in polymorphism, but it is an important thermodynamic variable that ranks alongside the temperature and the chemical potential for determining and causing changes in the states of matter and driving chemical reactions. Part of Bridgman's research involved measuring the effects of pressure on melting, and he described a few unusual substances including H2O, Bi and Ga that exhibited negative dTm/dP melting slopes. Later work revealed the existence of melting curve maxima, for elements such as Cs and Ba.1 In this review issue, M. C. Wilding et al. discuss the effects of high pressure on the structure and thermodynamic properties of liquids and amorphous solids. The discussion extends to the unusual phenomena of polyamorphism and density-driven liquid–liquid phase transitions that are related to the occurrence of negative dTm/dP slopes or maxima in the melting curves.29 A. San-Miguel describes the effects of pressure on various low-density open-framework cage structures and nanoparticles, that are stabilised in large part because of the surface free energy compared with that of fully-densified bulk materials. Here, the discussion can be extended into the “negative pressure” regime, where the system is placed under tensile strain.
Bridgman did not limit his researches to inorganic materials, but he carried out compression experiments on organic compounds and on biological molecules.8,28 The review by C. Pulham examines the pressure-induced polymorphism occurring in organic molecules including pharmaceuticals and explosives. F. Meersman et al. describe the effects of pressure on protein denaturation and folding transformations, along with the use of configurational energy landscapes30,31 to describe and understand the relationships between the different macromolecular structures including globular proteins vs. fibril formation. Since the discovery of abundant life forms at the deep ocean floor near spreading centres with their associated volcanic activity, as well as that concentrated around hydrothermally active sites on the continents, it has been estimated that the deep-surface biosphere may contain even more biomass than that expressed at the Earth's surface.32 It has been suggested that high-P,T environments could provide the setting for the emergence of early life on Earth. I. Daniel et al. review the geological setting for the early Earth, and they discuss the case for a deep ocean environment for the emergence of life. They also examine the effects of high pressure on simple organisms, and biological molecules and their reactions. The high pressure variable also has significant effects on higher biological functions. The review by A. Wlodarczyk et al. introduces various hyperbaric effects in neuroscience including the unusual phenomena of N2 and rare gas narcosis and pressure-reversal of anaesthesia. They also describe new approaches to the studying dynamic neuronal complex formation using voltage-sensitive dye fluorescence imaging, that can be readily adapted to in situ studies under high-pressure conditions.
Acknowledgements
High pressure research in the PFM group at UCL and at the RI has been supported by a Wolfson-Royal Society Research Merit Award Fellowship (2001–06) and EPSRC grants including Portfolio award EP/D504782 to PFM in collaboration with C. R. A. Catlow and P. Barnes. An EPSRC Senior Research Fellowship (EP/D07357X) has recently been awarded to PFM to develop the area of high pressure chemistry research.References
- L.-G. Liu and W. A. Bassett, Elements, Oxides, Silicates: High-Pressure Phases with Implications for the Earth's Interior, Oxford University Press, New York, 1986 Search PubMed.
- L. J. Parker, T. Atou and J. V. Badding, Science, 1996, 273, 95 CrossRef CAS.
- J. B. Goodenough, T. A. Kafalas and J. M. Longho, in Preparative Methods in Solid State Chemistry, ed. P. Hagenmuller, Academic Press, New York, 1972 Search PubMed.
- H. G. Drickamer and C. W. Frank, Electronic Transitions and the High Pressure Chemistry and Physics of Solids, Chapman & Hall, London, 1973 Search PubMed.
- High-Pressure Techniques in Chemistry and Physics, ed. W. B. Holzapfel and N. S. Isaacs, Oxford University Press, 1997 Search PubMed.
- R. J. Hemley and N. W. Ashcroft, Phys. Today, 1998, 51, 26–33 CrossRef CAS.
- W. Grochala, R. Hoffman, J. Feng and N. W. Ashcroft, Angew. Chem. Int. Ed., in press, 2006 Search PubMed.
- P. W. Bridgman, The Physics of High Pressure, G. Bell and Sons, London, 1931 Search PubMed.
- R. M. Hazen, The Diamond Makers, Cambridge University Press, Cambridge, 1999 Search PubMed.
- P. F. McMillan, Nat. Mater., 2005, 4, 715–718 CrossRef CAS.
- M. Eremets, High Pressure Experimental Methods, Oxford University Press, Oxford, 1996 Search PubMed.
- S. T. Weir, A. C. Mitchell and W. J. Nellis, Phys. Rev. Lett., 1996, 76, 1860–1863 CrossRef CAS.
- Ultrahigh-Pressure Mineralogy: Physics and Chemistry of the Earth's Deep Interior, ed. R. J. Hemley, Mineralogical Society of America, Washington D.C., 1998 Search PubMed.
- P. F. McMillan, Nat. Mater., 2002, 1, 19–25 CrossRef CAS.
- E. Wigner and H. B. Huntington, J. Chem. Phys., 1935, 3, 764–770 CrossRef CAS.
- A. R. Goni, K. Syassen, K. Strossner and M. Cardona, Phys. Rev. B: Condens. Matter, 1989, 39, 3178–3184 CrossRef CAS.
- M. I. Eremets, E. A. Gregoryanz, V. V. Struzhkin, H.-k. Mao and R. J. Hemley, Phys. Rev. Lett., 2001, 83, 2797–2800.
- V. V. Struzhkin, R. J. Hemley, H.-k. Mao and Y. A. Timofeev, Nature, 1997, 390, 382–384 CrossRef.
- K. Amaya, K. Shimizu and M. I. Eremets, Int. J. Mod. Phys. B, 1999, 13, 3623 CrossRef CAS.
- C. Narayana, H. Luo, J. Orloff and A. L. Ruoff, Nature, 1998, 393, 46–49 CrossRef CAS.
- P. Loubeyre, F. Occelli and R. LeToullec, Nature, 2002, 416, 613–617 CrossRef CAS.
- R. J. Hemley, Z. G. Soos, M. Hanfland and H.-k. Mao, Nature, 1994, 369, 84–387 CrossRef.
- M. Somayazulu, A. Madduri, O. Tschauner, P. F. McMillan, H.-k. Mao and R. J. Hemley, Phys. Rev. Lett., 2001, 87, 135504–135508 CrossRef CAS.
- D. Sihachakr and P. Loubeyre, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 134105 CrossRef.
- A. Goncharov, V. V. Struzhkin, H.-k. Mao and R. J. Hemley, Phys. Rev. Lett., 1999, 83, 1998–2001 CrossRef CAS.
- V. Iota, C. S. Yoo and H. Cynn, Science, 1999, 283, 1510–1513 CrossRef CAS.
- M. Santoro, F. Gorelli, R. Bini, G. Ruocco, S. Scandolo and W. A. Crichton, Nature, 2006, 441, 857–860 CrossRef CAS.
- P. W. Bridgman, Collected Experimental Papers, Harvard University Press, Cambridge, Massachussets, USA, 1964 Search PubMed.
- P. F. McMillan, J. Mater. Chem., 2004, 14, 1506–1512 RSC.
- D. J. Wales, Energy Landscapes, Cambridge University Press, Cambridge, 2003 Search PubMed.
- Configurational Energy Landscapes and Structural Transitions in Clusters, Fluids and Biomolecules, ed. P. F. McMillan and D. C. Clary, Philos. Trans. R. Soc. London, Ser. A, 2005, 363, 311–607 Search PubMed.
- D. S. Kelley, J. A. Baross and J. R. Delaney, Ann. Rev. Earth Planet. Sci., 2002, 30, 385–491 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
