High pressure effects in anaesthesia and narcosis
Agnieszka
Wlodarczyk
*abc,
Paul F.
McMillan
ab and
Susan A.
Greenfield
bc
aDepartment of Chemistry and Materials Chemistry Centre, Christopher Ingold Laboratories, University College London, 20 Gordon Street, London, UK WC1H 0AJ
bRoyal Institution of Great Britain, Davy-Faraday Research Laboratory, 21 Albemarle Street, London, UK W1X 4BS. E-mail: agnieszka@ri.ac.uk
cDepartment of Pharmacology and Oxford Centre for the Science of the Mind, Oxford University, Mansfield Road, Oxford, UK OX1 3QT. E-mail: agnieszka.wlodarczyk@pharm.ox.ac.uk
First published on 1st September 2006
Abstract
There is growing interest in determining the effects of high pressure on biological functions. Studies of brain processes under hyperbaric conditions can give a unique insight into phenomena such as nitrogen narcosis, inert gas anaesthesia, and pressure reversal of the effects of anaesthetic and narcotic agents. Such research may shed light on the action of anaesthetics, which remains poorly understood, and on the nature of consciousness itself. Various studies have established the behavioural response of organisms to hyperbaric conditions, in the presence or absence of anaesthetic agents. At the molecular level, X-ray crystallography has been used to investigate the incorporation of species like Xe in hydrophobic pockets within model ion channels that may account for pressure effects on neuronal transmission. New magnetic resonance imaging techniques are providing tomographic three-dimensional images that detail brain structure and function, and that can be correlated with behavioural studies and psychological test results. Such whole organ techniques are linked to the molecular scale via voltage-sensitive dye (VSD) imaging studies on brain slices that provide time-resolved images of the dynamic formation and interconnection of inter-neuronal complexes. The VSD experiments are readily adapted to in situ studies under high pressure conditions. In this tutorial review we review the current state of knowledge of hyperbaric effects on brain processes: anaesthesia and narcosis, recent studies at the molecular level via protein crystallography at high pressure in a Xe atmosphere, and we also present some preliminary results of VSD imaging of brain slices under hyperbaric conditions.
 Agnieszka Wlodarczyk | Dr Agnieszka Wlodarczyk is a postdoctoral researcher in the Davy-Faraday Laboratory at the Royal Institution of Great Britain and also in the Chemistry Department at University College London. She is also a visiting researcher in the Pharmacology Department at Oxford University. She graduated with an MS in Theoretical Physics in 1998 and completed her PhD in Biophysics in 2003 at Adam Mickiewicz University, Poznan, Poland. Since moving to London in 2003 she has started an interdisciplinary project on hyperbaric effects in neuroscience. Her research interests include optical methods to study biomolecules in solution and the dynamics of neuronal assemblies. |
 Paul F. McMillan | Paul F. McMillan has an established career in physical/solid state chemistry and physics with special emphasis on high pressure science, including designing high pressure cells and carrying out optical spectroscopy/imaging experiments in situ under high P conditions. He holds a joint post as Professor of Chemistry at UCL and in the Davy-Faraday laboratory at the RI. He has worked across the entire pressure range, from "extreme" high pressure conditions into the megabar range, to pressures of a few hundreds of atmospheres important in deep ocean biochemistry. His current research involves synthesis and study of novel solid state materials, including solids and liquid-state transitions, under high pressure–high temperature conditions. He has built programmes for in situ experiments on solids and liquids under "extreme" P–T conditions, using laser-heated diamond anvil cells to investigate materials at up to ∼1 Mbar and 3000-5000 °C. Other synthesis studies use "large volume" devices (1–20 GPa; 500–1800 °C); he is now developing "soft chemistry" approaches to inorganic–organic materials syntheses in the "lower" pressure range, between 1–5 kbar and up to a few hundred °C. |
 Baroness Greenfield | Baroness Greenfield is Director of the Royal Institution of Great Britain (the first woman to hold that position) and Professor of Pharmacology at the University of Oxford, where she leads a multidisciplinary team investigating neurodegenerative disorders. In addition she is Director of the Oxford Centre for the Science of the Mind, exploring the physical basis of consciousness. Her books include The Human Brain: A Guided Tour (1997), The Private Life of the Brain (2000), and Tomorrow's People: How 21st Century Technology Is Changing the Way We Think and Feel (2003). She has spun off four companies from her research, made a diverse contribution to print and broadcast media, and led a Government report on “Women In Science”. She has received 28 Honorary Degrees, Honorary Fellowship of the Royal College of Physicians (2000), a non-political Life Peerage (2001) as well as the Ordre National de la Legion d'Honneur (2003). In 2006 she was installed as Chancellor of Heriot-Watt University and voted ‘Honorary Australian of the Year'. |
Introduction
The science of the central nervous system (CNS) constitutes a vast research area that is evolving at various interfaces between the medical and behavioural sciences, experimental and observational biology, biochemistry and biophysics, and even extending into mathematics and philosophy, as we seek a general understanding of those collective phenomena that are associated with animal and human ‘consciousness’. The brain is a highly complex organ in which sensory information is transmitted and processed via electrochemical impulses throughout dynamically evolving networks of neuronal cells and their interconnections, to create signalling pathways and patterns that we recognise as perceptions, emotions and physiological responses. Recent advances in brain studies have resulted from the development of structural and functional imaging techniques including MRI (magnetic resonance imaging) and f- (functional) MRI. The images derived from these techniques applied during tasks or to patients exhibiting characteristic neurological symptoms are resulting in a remarkable new ‘science of the mind’, that permits correlations to be established between psychological observations, brain physiology, and the biochemistry and biophysics of neuroscience.The phenomenon of general anaesthesia is, by definition, a loss of consciousness and a reduced perception of and response to external stimuli, among mammals and other organisms, as a result of exposure to chemical agents. It is systematically applied in clinical practice, to suppress patient sensation and movement during highly traumatic and invasive surgery. Narcotics cause related physiological effects that involve altered states of consciousness, which are achieved voluntarily or involuntarily, by medical application or self-administration of drugs. Various anaesthetics have been identified and are administered with precision, according to empirical principles that have been developed through decades (and in some cases centuries) of medical experimentation. However, there is as yet no explanation for anaesthetic action.1–4
Major advances in neuroscience are now being made at a molecular level, through a combination of biochemistry and biophysics methods including electrophysiology, protein crystallography and sequencing, and recombinant DNA techniques and cell-expression studies of transmembrane ion channels, to gain a detailed understanding of the structure and properties of the ion channels involved in neuronal cell signalling and neurotransmitter receptor sites. The neurotransmitters themselves and the regions of the CNS in which they are active are identified by biochemical assays, combined with neurophysiology experiments. A large gap must be filled between those observations that link neurological chemistry to the electrophysiological responses at a molecular scale, and the large-scale properties of the dynamically evolving neuronal networks that are imaged by MRI and fMRI scanning, however. A link between the two can be provided by the use of voltage-sensitive dye (VSD) imaging techniques that are applied to brain slices during in vitro or in vivo experiments. These studies permit visualisation of the flow of electrochemical information though specified pathways in brain slices, via difference fluorescence spectroscopy with a time resolution appropriate to neuronal information transfer (e.g., 1–10 ms).5,6
Hyperbaric effects in neuroscience are well known. The physiological consequences of breathing compressed air and various gas mixtures have been documented since the advent of deep diving and as a result of studies of workmen exposed to sub-surface conditions in mines and other excavation sites. The unusual anaesthetic effects of chemically inert gases such as xenon, and nitrogen narcosis under hyperbaric conditions, have long provided an unresolved problem for neuroscience.7–10 Even more intriguing is the observation that the action of various narcotic and anaesthetic species can be reversed by application of high pressure conditions.11–13
This paper begins with a review of the pioneering experiments, which first demonstrated the link between high pressure and anaesthesia/narcosis. The next section is devoted to nitrogen narcosis and xenon anaesthesia, and these two phenomena are discussed together because the active species, namely N2 and Xe, gain their narcotic and anaesthetic properties respectively under increased pressure. There follows a review of recent experiments which have revealed that noble gases can occupy hydrophobic pockets buried within protein molecules; these findings may shed new light on the understanding of anaesthesia and narcosis. Next, we introduce the theories of anaesthesia and give possible explanations, as discussed in the literature, as to how pressure can reverse anaesthesia. The next section gives an introduction to a relatively new method in neuroscience in which optical imaging of neuronal voltage wave propagation in real time is studied using voltage-sensitive dyes. This technique has been used within a newly developed high pressure chamber for the in situ VSD imaging of rat hippocampal slices under hyperbaric conditions. The last section gives a summary and future directions. An introduction to basic concepts and techniques in neuroscience can be found in standard texts.14
Recognition of hyperbaric effects on anaesthesia and narcosis: luminescent bacteria, firefly luciferase, and pressure reversal of narcosis in tadpoles and newts
Early studies of anaesthetic action at a molecular level were limited by a lack of detailed understanding of the complicated structure and biochemistry associated with neurotransmitter receptor sites and ion channels. However, a useful model was discovered for anaesthesia and narcosis among the light-emitting proteins and enzymes present within luminous bacteria and insect species, such as firefly luciferase15,16 These had well-determined crystal structures that were much simpler than the neurotransmitter receptors or ion channel protein complexes, and they could be used as convenient models for understanding anaesthetic action at the molecular level. The action of luminescent proteins was inhibited, or could be enhanced in certain cases, by the action of molecular species that are well known anaesthetics.17 Hyperbaric conditions resulted in extinction of the chemiluminescence observed among bacterial enzymes, as well as from luciferase, suggesting a new model for understanding anaesthesia and narcosis, as well as leading to new links between high pressure research and neuroscience.15,18,19 Studying hyperbaric effects on bacterial/luciferase luminescence quickly became an important component of studies directed at understanding anaesthetic function and activity.13,15,17 Although certain studies have questioned the existence of the pressure reversal effects observed for luciferase,20 later work appears to confirm pressure reversal of the kinetic effects on luminescence that are associated with narcotic action.19Following early observations of extinction of the luminescence from firefly luciferase and luminescent bacteria under hyperbaric conditions,21 the first direct evidence for pressure reversal of anaesthesia/narcosis was obtained from studies of tadpoles treated with ethanol.11 In a now-classic study, young larvae of Rana sylvatica were placed in a 3–6% ethanol solution in water (i.e., similar to beer) where they became narcotised, in that they ceased moving spontaneously and they no longer responded to external stimuli. They were then transferred to a hyperbaric chamber equipped with observation windows. As the pressure was raised to 2000–5000 psi (130–330 atm) the animals resumed their normal swimming behaviour, indicating that the effects of alcohol had been antagonised by the application of high hydrostatic pressure. A similar pressure reversal effect was observed for tadpoles narcotised using urethane (0.08 M), but not for n-amyl carbamate (0.001 M), showing that the effect is not completely general for all anaesthetic or narcotic species. Johnson and Flagler made an interesting observation on unnarcotised tadpoles under hyperbaric conditions. With increasing pressurisation, the animals became more active, indicating that pressure alone has an influence on neuronal excitability. The maximum hyperactivity occurred at around 2000 psi (1 atm = 14.7 psi), and thereafter it decreased. By 5000 psi, all motion had ceased. As noted by Johnson and Flagler, similar behaviour had been observed previously for other aquatic species under hyperbaric conditions.
In studies by Lever et al., narcosis induced in Italian crested newts by 34 atm of N2 (see next section) was found to be antagonised by increasing the pressure to 140 atm using He gas. Newts were also submerged in liquids and anaesthetised with solutions of sodium pentobarbitone, ether, halothane or butanol, and the pressure was applied mechanically in the absence of a gas phase. In both series of hyperbaric experiments, increasing the total pressure over a range up to 140 atm was found to antagonise the anaesthetic effects.13 Observations of pressure reversal effects were then extended to mammals. Mice were first narcotised by exposure to 45 atm of N2, and then subjected to hyperbaric compression with He. Analogous results were obtained with sodium pentobarbitone.13
In more recent work, specialised hyperbaric chambers were developed to carry out not only behavioural studies of whole animals, but to enable detailed neuronal electrophysiology measurements at high pressure. Using the new chambers, continuous intracellular recording signals (ICRs) could be obtained from, e.g., mammalian neurons within brain slices at high pressure.12,22–24 The methods are readily extended to studies of individual receptor sites and transmembrane ion channels, that are conveniently expressed via recombinant DNA techniques within the large eggs (i.e., oocytes) of the frog Xenopus. Patch-clamp techniques that enable a direct measurement of an ion current passing through ion channel proteins are implemented in the high pressure chamber.12
Within the hyperbaric chambers designed for electrophysiology experiments, gas pressures up to approximately 12 atm absolute (i.e., 12 ata, rather than pressures relative to ambient conditions) are applied using various pure or mixed gases (e.g., compressed air, He, He–O2 ‘heliox’ mixtures) that can be pumped into the chamber or derived from high pressure gas cylinders. Important external parameters, such as the bath and tissue slice temperature, the O2 partial pressure (pO2), and the pH, are all constantly monitored during the hyperbaric voltage recording experiments. Such studies give detailed information on features of the neuronal electrochemical activity, including membrane conductance, synaptic potentials, and firing rate that can be correlated with the presence or absence of anaesthetic or narcotic species under hyperbaric conditions.
In addition to determination of pressure reversal effects, the electrophysiology experiments allow us to determine the tissue and cellular responses to hyperbaric conditions elicited by different pressurising gases, to identify various types of barosensitivity, and also to study the phenomenon of inert gas narcosis and O2 sensitivity in the same neuron.12,22–24
The new apparatus and experimental strategies have been applied to study the binding of alcohols, especially ethanol, to receptor sites within the CNS.12,23,24 Exposure to 12 ata heliox was found to antagonise ethanol potentiation of GABAA-activated Cl− uptake in brain membranes from three strains of genetically modified mice. At the range of alcohol concentrations used in the study (25–200 mM), the gas-applied high pressure conditions caused the behavioural and anaesthetic effects of ethanol to be reversed.23 These results provided new evidence that ethanol potentiation of the GABAA receptor (i.e., GABAAR) site is a key function of ethanol that leads to its behavioural and psychological consequences.23 Two years later the same authors extended their study to the biochemical level using glycine receptors (GlyR) expressed in Xenopus oocytes.12 Those pioneering results indicated that pressure directly and selectively antagonises ethanol potentiation of the α1GlyR function in a reversible and concentration- and pressure-dependent manner,12 and that alcohols may act by a common mechanism on ligand-gated ion channels.12,23
Although alcohol (ethanol) clearly exhibits pressure reversal effects, it is perhaps not an ideal test case to develop detailed models for hyperbaric effects on anaesthesia and narcosis, because of the biochemical and neurophysiological complexity of the drug action.25 For many years, it has been debated whether the primary mode of action of alcohol occurred within the lipid cell membrane, by disrupting the fatty acid structural arrangement or changing the membrane volume or its curvature, or more specifically at protein sites associated with receptors or transmembrane ion channel complexes.25 During the past two decades, there has been growing evidence that the most important sites for ethanol potentiation lie within neurotransmitter receptors or ion channels.12,23,25 Specific alcohol binding sites within protein complexes that provide useful models of the transmembrane ion channels and receptor sites are now being identified, by a combination of biochemical studies combined with X-ray crystallography and NMR structure determination techniques.23,26–28 The primary binding site for alcohols, including ethanol, is thought to occur deep within the GABAA receptor, thus modifying the inhibitory GABA-gated entry of Cl− ions into the cell29,30 (Fig. 1). However, glutamate (NMDA) receptors are also thought to form important targets for alcohol binding within the CNS, for example those for AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid) and/or kainate that mediate fast excitatory transmission within the CNS. Ethanol binding likely occurs at several different sites within various ion channels and neuroreceptor sites to result in the neurological and physiological consequences, and membrane lipid solubility likely also plays a role, under variable concentration- and pressure-dependent regimes.
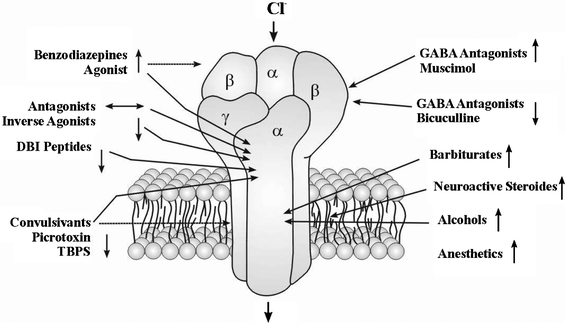 | ||
| Fig. 1 A general structural model for the GABAA receptor redrawn from Cooper et al.29 The GABAA complex is a pentamer of two α, two β and one γ subunits, formed by α-helical portions of a single transmembrane protein that loops back and forth throughout the cell membrane. It is a ligand-gated Cl− channel that has an inhibitory action on neurotransmission. The receptor binds the agonist GABA and neuroactive species such as muscimol, and also the antagonist bicuculline on the α and β subunits. Also there are binding sites on the receptor for barbiturates, ethanol, and on the α subunit for the benzodiazepine family of narcotics. The primary binding site for alcohols including ethanol is thought to occur deep within the GABAA receptor.29,30 All these substances potentiate the channel-opening action of GABA on the GABAA receptor. | ||
Nitrogen narcosis and xenon anaesthesia
A second large area developed at the interface between neuroscience and high pressure studies concerns the narcotic/anaesthetic effects that are associated with ‘chemically inert’ species such as N2, Xe, and Kr.10,31 Nitrogen narcosis occurring under high pressure conditions (e.g., P = 5–12 bar) has been recognised since the earliest studies of human and animal physiological responses under deep-sea diving conditions, that result in euphoria, dizziness or loss of concentration. This phenomenon is distinct from the ‘bends’ and decompression sickness that result from physical dissolution of the molecular gas into body fluids at high pressures, followed by rapid exsolution during decompression. It is now thought that the altered state of consciousness involved in N2 narcosis could result from binding of the neutral, non-polar and chemically unreactive molecule to specific sites within the ion channels associated with neurotransmission. Recent biochemical and crystallographic studies could indicate that N2 binding might occur at hydrophobic sites, such as those present within ion channels associated with glutamate receptors.9The surprising general anaesthetic effect of the noble gases Xe and Kr has long proved a highly puzzling biochemical/physiological phenomenon.10,31–33 Xenon is now well known to produce anaesthesia in humans and other mammals, with apparently minimal side effects, and it has significant potential for clinical applications, despite the high cost of the gas.34 It was recently determined that anaesthetic use of Xe results in remarkable cardiovascular stability, rapid onset and offset of its action resulting from its extremely low blood–gas partition coefficient, neuroprotection and profound analgesia. No evidence of toxicity and no mutagenic or carcinogenic properties were found in several studies.34 The minimum alveolar concentration in humans (MAC†) of Xe is 0.6–0.7 atm. The MAC for Xe in other organisms is higher: for monkeys and dogs it ranges between 0.9–1.2 atm, and in rats it is ∼1.6 atm.10 Kr shows anaesthetic effects at higher pressures than Xe; He and Ne do not show any anaesthetic action before the onset of convulsions (followed by death) at ∼85 atm (He) and 90–130 atm (Ne) in rats and mice. The sites occupied by the noble gas atoms (and N2) are not well known at present. However, biochemical studies combined with crystallography and molecular dynamics simulations indicate that Xe, Kr and N2 could occupy hydrophobic sites or pockets within ion channels such as those associated with the excitatory glutamate (NMDA) neurotransmitter receptor complex.9
Hydrophobic pockets
A further unexpected link between the high pressure variable, anaesthesia/narcosis studies and the role of hydrophobic sites developed within protein complexes arose from macromolecular X-ray crystallography. A classical challenge in solving the structures of crystalline solids from X-ray diffraction data is due to the so-called ‘phasing’ problem that arises from the combination of X-ray waves modulated by the atomic scattering factors to yield the observed intensity patterns of the diffracted signals. A traditional technique for overcoming this problem is the ‘heavy atom’ method, in which the anomalously large X-ray scattering from an atomic species with large atomic number is introduced into the specific sites within the molecule to be determined, hopefully with minimal perturbation of the surrounding structure. Such heavy-atom techniques have been applied to resolve the crystal structure of proteins and other biologically important macromolecules.35 One of the main challenges is to identify appropriate heavy atoms that do not interact chemically with the protein substrate, and that do not substantially perturb its structure. An elegant solution to this problem was found by using Xe or Kr atoms introduced under high pressure conditions into the macromolecular complex.36–38 During that work, it was found that the noble gas atoms occupied specific hydrophobic sites within the protein substrates, including regions that modelled the hydrophobic pockets that are expected to occur within ion channels associated with neurotransmission36,38–40 (Fig. 2). It is also interesting to note that the 129Xe isotope is NMR active and is susceptible to a greatly enhanced NMR signal by laser-polarisation experiments.41 | ||
| Fig. 2 The crystal structure of unligated maltose binding protein with xenon, deduced using 129Xe NMR spectroscopy.41 This type of Xe binding site within hydrophobic pockets developed in the transmembrane protein complexes is confirmed and established by X-ray crystallography, using the heavy-atom method. | ||
Theories of anaesthesia and narcosis and hyperbaric effects
The large group of substances that cause general anaesthetic effects comprises a wide range of chemically and structurally dissimilar molecules (e.g., alcohols, esters, ketones, aldehydes, ethers, hydrocarbons, noble gases, etc.). The wide diversity of chemical species that produce similar results means that the overall effects of anaesthesia could be considered to be induced by non-chemically specific interactions. The question of specific sites at which various anaesthetics act is still a matter of considerable debate, however. In fact, it now appears highly unlikely that any single detailed theory of anaesthetic action could possibly explain all anaesthetic/narcotic effects, although the overall consequences could appear similar at the level of a whole organism. We can roughly classify various anaesthetic theories according to the assumed site and mode of their action within the neuronal cells.(i) Lipid theories
One of the earliest quantitative theories of anaesthesia is attributed to Meyer and Overton, based on their discovery that the potencies of various anaesthetic species are generally proportional to their solubility in fatty substances.42,43 Anaesthetic potency is usefully defined as the reciprocal of the anaesthetising ED50 partial pressure for gases, or the corresponding reciprocal of the molar anaesthetising ED50 concentration for soluble anaesthetics. The fatty liquid solubility was calibrated in olive oil at standard pressure for gases, or determined by the oil–water distribution coefficient for water-soluble species. The Meyer–Overton observations cover over five orders of magnitude, and are generally recognised as providing one of the most successful correlations in all of the physical sciences. The correlation is improved in quality and the range of anaesthetics it describes if octanol or a fully hydrated fluid lipid bilayer is used instead of olive oil. Those findings led to a general theory that anaesthetics dissolve in the lipid fraction of the cell membrane, thus altering the physiological properties.43 This brought the study of neuronal lipid bilayers to the fore as a primary target site of anaesthetic action. Modern variants of the lipid theory developed related membrane properties that could be relevant to anaesthesia, including volume expansion and ‘lateral surface pressure’, membrane fluidity and thickness, and surface tension effects.44 Such an altered state of the membrane lipids might then change the activity and function of integral membrane proteins, including ion-channels, thereby inducing anaesthesia. Modern lipid theories often postulate such an indirect mechanism for the occurrence of anaesthesia/narcosis. The main drawback of these models lies in the observation that the anaesthetic concentrations needed to produce relevant changes in membrane lipid properties would be highly toxic to the organism.45(ii) Protein theories for anaesthetic action
Theories for anaesthetic action based at protein sites also go back to the late 19th century; however, the apparent success of the Meyer–Overton correlation in explaining anaesthetic properties meant that lipid-based models have dominated thinking in the field for many decades. The remarkable finding that the soluble protein of firefly luciferase could provide a good model for anaesthetic action represented a key advance in the field.46 The result led to the discovery that the action of various general anaesthetics on luciferase protein gave a similar linear correlation between the ED50 for luciferase inhibition and that for general anaesthesia, that was analogous to the Meyer–Overton plot.47,48 Detailed analysis of anaesthetic–luciferase interactions led to the suggestion that anaesthetic molecules compete with substrate luciferin molecules for binding to the protein hydrophobic pocket.47,48 This discovery of a luciferase response to anaesthetics was remarkable, because most soluble proteins had been found to be insensitive to these species, or that they respond to only a few of them, at MAC concentrations.7 It is now thought that ion channels and neurotransmitter receptor sites formed from protein complexes embedded within the neuronal cell membranes constitute the primary sites of anaesthetic action.7,8,27 Discovery of the stereospecificity of certain anaesthetics and their optical isomers, which are equally soluble in lipids, supports a protein-based theory of anaesthetic action.49(iii) Macromolecule–water interface theories
Many of the gas phase species that exhibit anaesthetic properties also form crystalline hydrates. Comparison of the anaesthetising partial pressure with the equilibrium partial pressure above the corresponding hydrate crystals at 0 °C gives a linear relation analogous to the Meyer–Overton plot. This relationship was noted independently by Pauling and by Miller in 1961.50,51 A new theory was proposed, in which the site of general anaesthetic action would lie within the aqueous phase of the central nervous system and that the anaesthetised state is due to clathrate formation at the membrane surface. However, typical gas clathrate hydrates are not stable under physiological pressure–temperature conditions. To support the model, additional factors were considered that could increase the clathrate stability in a physiological environment. Miller postulated the primary target of anaesthetic action at an interface between water and the membrane surface, or between highly ordered ‘ice-like’ states of inter-cellular water and at the ‘surface’ of macromolecules such as proteins, lipids, polysaccharides, etc.51 Anaesthetic gas molecules might then occupy structure-determining cavity sites within dynamic ‘ice-like’ liquid water clusters. This approach can be regarded as a precursor of developing theories that stress the influence of anaesthetics on structuring the water layer at the ‘surface’ of macromolecules e.g.ref. 46. Such theories combine aspects of both lipid and protein theories of anaesthetic action.(iv) Pressure reversal of anaesthesia
The observed pressure reversal of anaesthesia and narcosis is one of the most intriguing features of the anaesthetic state. To date, there are two main types of theoretical explanation for this effect. According to one view, anaesthetics and pressure act on different molecular targets, and the pressure reversal is a mere consequence of a general stimulation brought on by pressure overcoming the general depression of physiological and mental activity caused by the anaesthetic species. Indeed, although the anaesthetic potencies of various substances are similar among a wide range of organisms, the values of the pressures required to reverse anaesthesia vary considerably between organisms, and in some cases the pressure reversal is not observed at all.11,13 An alternative point of view assumes that pressure and anaesthesia act antagonistically at the same molecular sites, and therefore the pressure reversal effect is intimately related to a general mechanism of anaesthetic action. Since one of the most obvious effects of the increase of pressure at constant temperature is a volume reduction, some authors have argued that anaesthetics act by increasing the local volume contribution of some crucial target in the nervous system. That is the basis of the ‘critical volume’ hypothesis52 and its variants.53 From the point of view of the lipid theories, the pressure reversal effect is a consequence of the observation that higher pressures reverse many anaesthetic-induced perturbations of lipid bilayers. However those pressures are usually much lower than the pressures needed to reverse anaesthesia in whole organisms.13 Protein theories, that assume direct binding of anaesthetics to protein sites within ion channels or at receptor sites, account for pressure reversal through dislocation/dissociation of the anaesthetic molecules from their usual targets, or modification of the target action.Optical studies of voltage wave propagation and neuronal information transfer using voltage-sensitive dyes
What defines the central nervous system, in terms of its action and functionality, is not a property of a single neuron but the collective behaviour of ‘neuronal complexes’ or assemblies that are formed by dynamic interaction of neurons within the whole neuronal network.54 Studies on the intact brains of whole animals are influenced by many different physiological affects, and there is little hope of deconstructing the behaviour in terms of microscopic neuronal properties. This is why the study of neuronal assemblies at the mesoscale leads to the definition of a fundamental distance scale in neuroscience research. Recent studies now being carried out are highlighting the importance of transient assembly formation involving 105–108 inter-neuronal connections formed dynamically during the development and passage of an electrical wave pattern in the CNS; these dynamically-formed large scale complexes are thought to determine various states of consciousness and memory. Direct observation of the transient formation of such neuronal assemblies is achieved using recently developed fast CCD two-dimensional detection systems, via fluorescence microscopy on brain slices treated with voltage sensitive dyes (VSD imaging).5,6VSDs take their name from their ability to undergo changes in their electronic structure and consequently their fluorescence spectra in response to changes in the surrounding electric field. One of the most widely used VSDs for brain slice studies is di-4-ANEPPS (D-1199, Molecular Probes Inc.) which is sensitive to submillisecond membrane potential changes and ±0.1 mV changes in fluorescence intensity (Fig. 3). VSDs are generally lipophilic molecules, which stain the neural cell membranes. Other important properties include photostability and non-toxicity to the neuronal tissue. Their optical response is sufficiently fast to detect transient potential changes in neuronal cells. During passage of the electrical signal within the neurons the change in the intensity of fluorescence (ΔF) is measured in each pixel of the CCD image, relative to the initial intensity of fluorescence (F). The optical response recorded by VSD fluorescence thus tracks effectively, in real time, the passage of a voltage wave throughout the neuronal system. VSD imaging provides a useful way of observing the formation and flow of large-scale dynamic neuronal complexes in real time, at a distance scale that is intermediate between that of the molecular interactions at the membrane and synapse, and those at the whole-organ or whole-animal level probed by magnetic resonance imaging or physiological/psychology studies. A typical VSD imaging system consists of a stage with a chamber to mount the brain slice, an excitation light source, and a CCD camera for data collection.6 The sample stage is equipped with electrodes for stimulation of the neuronal signal that can also be used for electrophysiological recording. The excitation light is provided by a halogen lamp (150 W), introduced into the microscope system by a light-guide with an appropriate condenser lens. The fluorescence signal is collected by and captured on the CCD sensor of the optical imaging system (Fig. 4).
 | ||
| Fig. 3 The structure of di-4-ANEPPS dye molecule used in VSD imaging. | ||
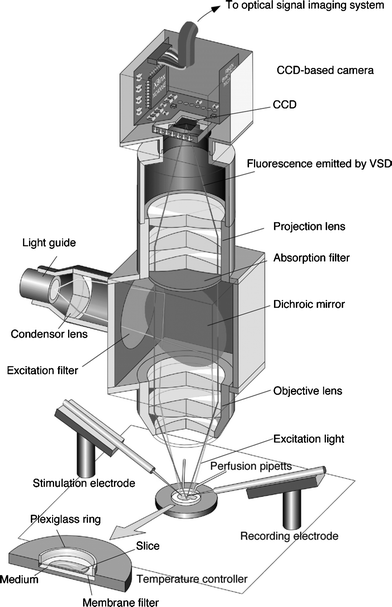 | ||
| Fig. 4 A schematic illustration of the CCD camera system, optics and set-up used in VSD imaging studies,6 including under hyperbaric conditions. | ||
We recently developed an experimental pressure chamber to conduct in vitro VSD imaging experiments of the flow of voltage-regulated information in neuronal complexes under hyperbaric conditions (Fig. 5). Preliminary results indicate that under hyperbaric conditions (P = 32 atm) the neuronal voltage response is much stronger, and the signal propagation along the CA3–CA1 Schaeffer collateral is considerably extended (Fig. 6).
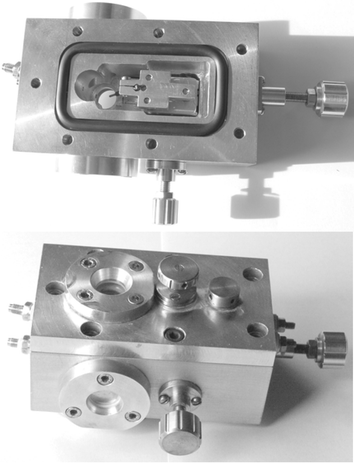 | ||
| Fig. 5 Top and bottom: images of the hyperbaric cell for VSD imaging studies of neuronal signal propagation. Top: the specially designed carriage for x-, y-, z-manipulation of the electrode assembly is seen, with the electrode poking down into the circular brain slice mounting area. Bottom: the cell assembly is shown with the viewing windows in place. Pumping of artificial cerebrospinal fluid (aCSF) to achieve hyperbaric conditions is achieved through the inlet and outlet connections on the right, and electrode manipulation occurs by turning the knurled brass knobs. The cell fits underneath the VSD fluorescence microscopy imaging instrument. | ||
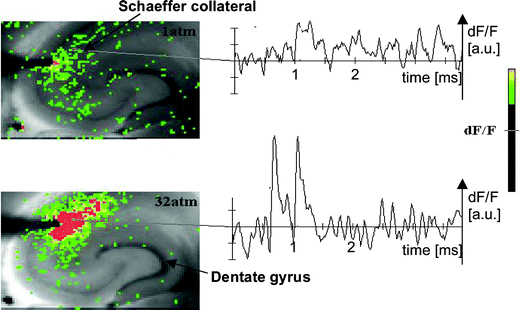 | ||
| Fig. 6 A fluorescent image of a rat hippocampal slice obtained using the VSD method at ambient pressure and 32 atm respectively in the high pressure cell. Still images of neuronal transient assembly formation on a Schaeffer collateral pathway of a rat hippocampal slice stained with VSD are shown. The overall image is 2 × 3 mm and each pixel provides an optical trace of differential fluorescence dF/F over time. The maximum dF/F signals are superimposed on the hippocampus image with the excitation electrode, The Schaeffer collateral and dentate gyrus are easily recognised on the slice. The amplitude of the optical signal represented in pseudo-colours indicates the degree of membrane depolarisation. Two electrical pulses of amplitude 60 V lasting 0.1 ms each, separated from each other by 60 ms, triggered spreading of the neuronal signal within the brain slice. The depolarisation started at the stimulating electrode, with the first excitation pulse occurring 100 ms after starting the measurement, and spread along the CA3–CA1 Schaeffer collateral pathway. Under hyperbaric conditions (P = 32 atm), the neuronal voltage response is much stronger, and the signal propagation along the CA3–CA1 Schaeffer collateral is considerably extended. | ||
Conclusions
There is still no accepted general theory of how anaesthetics work, in spite of the enormous efforts in clinical and laboratory research carried out for many decades. After the recognition of hyperbaric effects on anaesthesia and narcosis and recognition of neurological syndromes related to human exposure to the high pressure environment there are new scientific findings which link high pressure research with neuroscience. Various anaesthetic theories according to the assumed site and mode of their action within the neuronal cells have been proposed. The lipid theory based on the Meyer–Overton correlations suggests that the neuronal lipid bilayer is the primary target site of anaesthetic action. However, the anaesthetic concentrations needed to produce relevant changes in membrane lipid properties would be highly toxic to the organism. It is now thought that ion channels and neurotransmitter receptor sites formed from protein complexes embedded within the neuronal cell membranes constitute the primary sites of anaesthetic action. Discovery of the stereospecificity of certain anaesthetics and their optical isomers, that are equally soluble in lipids, supports a protein-based theory of anaesthetic action. The structures of CNS neuroreceptors are still not completely solved, including their precise location in the neuronal membrane. Recent studies have revealed that Xe, like other noble gases with anaesthetic potencies under elevated pressure, can occupy hydrophobic pockets buried within protein molecules. These findings give new insights into the understanding of anaesthesia and narcosis. New studies using voltage-sensitive dye imaging provide insights into the dynamic formation of neuronal complexes that may permit a link between molecular-scale and animal studies. VSD imaging can be readily carried out in situ under hyperbaric conditions.Acknowledgements
Our experimental work to date to develop the hyperbaric cell for VSD measurements has been supported by a Wolfson-Royal Society Research Merit Award Fellowship to PFM and an EPSRC Portfolio grant EP/D504782 to PFM in collaboration with C. R. A. Catlow and P. Barnes. These awards also support AW's stipend. The VSD imaging experiments, along with animal and laboratory fees, are supported by a grant awarded from the Templeton Foundation to SAG to establish the Oxford Centre for the Science of the Mind (OXCSOM). We thank two anonymous referees for their comments that greatly improved the manuscript.References
- N. P. Franks and W. R. Lieb, Nature, 1978, 274, 339 CAS.
- N. P. Franks and W. R. Lieb, Nature, 1982, 300, 487 CrossRef CAS.
- N. P. Franks and W. R. Lieb, Nature, 1994, 367, 607 CrossRef CAS.
- N. P. Franks and W. R. Lieb, Toxicol. Lett., 1998, 100–101, 1 CrossRef CAS.
- A. Grinvald, R. D. Frostig, E. Lieke and R. Hildesheim, Physiol. Rev., 1988, 68, 1285 Search PubMed.
- T. Tominaga, Y. Tominaga, H. Yamada, G. Matsumoto and M. Ichikawa, J. Neurosci. Methods, 2000, 102, 11 CrossRef CAS.
- N. P. Franks and W. R. Lieb, Nature, 1982, 300, 487 CrossRef CAS.
- N. P. Franks and W. R. Lieb, Nature, 1994, 367, 607 CrossRef CAS.
- J. R. Trudell, D. D. Koblin and E. I. Eger, Anesth. Analg., 1998, 87, 411 Search PubMed.
- D. D. Koblin, Z. Fang and E. I. Eger, Anesth. Analg., 1998, 87, 419 Search PubMed.
- F. H. Johnson and E. A. Flagler, Science, 1950, 112, 91 CrossRef CAS.
- D. L. Davies, J. R. Trudell, S. J. Mihic, D. K. Crawford and R. L. Alkana, Alcohol.: Clin. Exp. Res., 2003, 27, 743 Search PubMed.
- M. J. Lever, K. W. Miller, W. D. Paton and E. B. Smith, Nature, 1971, 231, 368 CrossRef CAS.
- E. R. Kandel, J. H. Schwarz and T. M. Jessell, Principles of Neural Science, McGraw-Hill, New York, 2000 Search PubMed.
- N. P. Franks, A. Jenkins, E. Conti, W. R. Lieb and P. Brick, Biophys. J., 1998, 75, 2205 CrossRef CAS.
- M. J. Halsey and E. B. Smith, Nature, 1970, 227, 1363 CrossRef CAS.
- R. Dickinson, N. P. Franks and W. R. Lieb, Biophys. J., 1993, 64, 1264 CrossRef CAS.
- I. Ueda, Keio J. Med., 2001, 50, 20 Search PubMed.
- I. Ueda, H. Minami, H. Matsuki and T. Inoue, Biophys. J., 1999, 76, 478 CrossRef CAS.
- G. W. J. Moss, W. R. Lieb and N. P. Franks, Biophys. J., 1991, 60, 1309 CrossRef CAS.
- F. H. Johnson, H. H. Eyring and R. B. Williams, J. Cell Comp. Physiol., 1942, 20, 269 Search PubMed.
- J. B. Dean and D. K. Mulkey, J. Appl. Physiol., 2000, 89, 807 Search PubMed.
- D. L. Davies and R. L. Alkana, Alcohol.: Clin. Exp. Res., 2001, 25, 1098 Search PubMed.
- D. L. Davies and R. L. Alkana, Eur. J. Pharmacol., 2003, 469, 37 CrossRef CAS.
- R. W. Peoples, C. Li and F. F. Weight, Annu. Rev. Pharmacol. Toxicol., 1996, 36, 185 CrossRef CAS.
- D. L. Davies, T. K. Machu, Y. Guo and R. L. Alkana, Alcohol.: Clin. Exp. Res., 2002, 26, 773 Search PubMed.
- S. J. Mihic and R. A. Harris, Neurotransmissions, 1997, 13, 1 Search PubMed.
- S. W. Kruse, R. Zhao, D. P. Smith and D. N. Jones, Nat. Struct. Biol., 2003, 10, 694 CrossRef CAS.
- J. R. Cooper, F. E. Bloom and R. H. Roth, The Biochemical Basis of Neuropharmacology, Oxford University Press, Oxford, 2003 Search PubMed.
- R. F. Thompson, The Brain: A Neuroscience Primer, Worth Publishers, New York, 3rd edn, 2000 Search PubMed.
- N. P. Franks, R. Dickinson, S. L. de Sousa, A. C. Hall and W. R. Lieb, Nature, 1998, 396, 324 CrossRef CAS.
- S. C. Cullen and E. G. Gross, Science, 1951, 113, 580 CrossRef CAS.
- J. H. Lawrence, W. F. Loomis, C. A. Tobias and F. H. Turpin, J. Physiol., 1946, 105, 197 CAS.
- R. D. Sanders, D. Ma and M. Maze, Br. Med. Bull., 2005, 71, 115 Search PubMed.
- T. J. Boggon and L. Shapiro, Structure, 2000, 8, R143 CrossRef CAS.
- M. Schiltz, R. Fourme and T. Prange, Methods Enzymol., 2003, 374, 83 CAS.
- M. L. Quillin, W. A. Breyer, I. J. Griswold and B. W. Matthews, J. Mol. Biol., 2000, 302, 955 CrossRef CAS.
- A. E. Eriksson, W. A. Baase, X.-J. Zhang, D. W. Heinz, M. Blaber, E. P. Baldwin and B. W. Matthews, Science, 1992, 255, 178 CrossRef CAS.
- M. Brunori, B. Vallone, F. Cutruzzola, C. Travaglini-Allocatelli, J. Berendzen, K. Chu, R. M. Sweet and I. Schlichting, Proc. Natl. Acad. Sci. U. S. A., 1999, 97, 2058.
- V. N. Malashkevich, R. A. Kammerer, V. P. Efimov, T. Schulthess and J. Engel, Science, 1996, 274, 761 CrossRef CAS.
- S. M. Rubin, M. M. Spence, I. E. Dimitrov, E. Janette Ruiz, A. Pines and D. E. Wemmer, J. Am. Chem. Soc., 2001, 123, 8616 CrossRef CAS.
- H. H. Meyer, Arch. Exp. Path. Pharmak., 1899, 42, 109 Search PubMed; E. Overton, Studien uber die Narkose, Fischer, Jena, 1901 Search PubMed.
- H. H. Meyer, Arch. Exp. Pathol. Pharmakol., 1899, 109.
- N. P. Franks and W. R. Lieb, Nature, 1987, 328, 113 CrossRef CAS.
- N. P. Franks and W. R. Lieb, Alcohol Alcohol. Suppl., 1987, 1, 139 Search PubMed.
- I. Ueda, J.-S. Chiou, P. R. Krishna and H. Kamaya, Biochim. Biophys. Acta, 1994, 1190, 421 CAS.
- N. P. Franks and W. R. Lieb, Nature, 1985, 316, 349 CAS.
- N. P. Franks and W. R. Lieb, Nature, 1984, 310, 599 CAS.
- N. P. Franks and W. R. Lieb, Science, 1991, 254, 427 CrossRef CAS.
- L. Pauling, Science, 1961, 134, 15 CrossRef CAS.
- S. L. Miller, Proc. Natl. Acad. Sci. U. S. A., 1961, 47, 1515 CrossRef CAS.
- K. W. Miller, W. D. Paton, R. A. Smith and E. B. Smith, Mol. Pharmacol., 1973, 9, 131 CAS.
- M. J. Halsey, B. Wardley-Smith and C. J. Green, Br. J. Anaesth., 1978, 50, 1091 CrossRef CAS.
- S. A. Greenfield and T. F. Collins, Prog. Brain Res., 2005, 150, 11 Search PubMed.
Footnote |
| † MAC is defined as the minimum alveolar concentration of an inhalation anaesthetic, at one atmosphere pressure, that produces immobility in 50% of the subjects exposed to a noxious stimulus. Thus, MAC corresponds to the effective dose that anaesthetised half of the subjects (i.e. ED50). |
| This journal is © The Royal Society of Chemistry 2006 |
