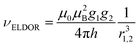PELDOR study on the tyrosyl radicals in the R2 protein of mouse ribonucleotide reductase†
Daniele
Biglino
,
Peter P.
Schmidt
,
Edward J.
Reijerse
and
Wolfgang
Lubitz
*
Max Planck Institute for Bioinorganic Chemistry, D-45470, Mülheim an der Ruhr, Germany. E-mail: lubitz@mpi-muelheim.mpg.de
First published on 21st November 2005
Abstract
Pulse electron–electron double resonance (PELDOR) has been employed to measure the distance between the putative tyrosyl radicals in the two halves of the R2 subunit from mouse ribonucleotide reductase. The results provide experimental evidence that the active, tyrosyl radical containing mouse R2 subunit forms a homodimeric form in solution. The distance between the two tyrosyl radicals present in the dimer was determined to be 3.25 ± 0.05 nm.
1. Introduction
Ribonucleotide reductase (RNR) is the enzyme that catalyzes the reduction of nucleotides to deoxynucleotides in all organisms, a fundamental step in DNA replication and repair.1,2 RNR enzymes can be divided into three classes (I, II, III) depending on the active site, quaternary structure, and oxygen sensitivity;1,2 class I is further divided into subclasses Ia and Ib depending on differences in allosteric regulation.3 RNR from mouse, as RNR from all mammals and several bacteria, belongs to class Ia. Class Ia RNR has a tetrameric structure, α2β2, formed by two homodimeric subunits: R1 (αα) and R2 (ββ). R1 contains the substrate binding active site. In R2 a stable tyrosyl radical (Y˙) and an antiferromagnetically coupled diiron(III) center is accommodated that are essential for activity. The Y˙ and the active site are separated by about 3.0 nm. In Fig. 1 the structure of the RNR from E. coli is shown.4 The tyrosyl radical and the diiron center site are highlighted. Electron paramagnetic resonance (EPR) techniques have been extensively employed to study both the Y˙ structure and the oxidation state of the iron center during the reconstitution process of RNR.2,5–7 The cw-EPR spectrum of the tyrosyl radical present in mouse R2 is shown in Fig. 2 together with the structure of the Y˙ radical. The main spectral feature is the large splitting arising from the hyperfine coupling of the unpaired electron with one of the hydrogens in β-position (Ax = 2.14, Ay = 1.90, Az = 2.15 mT), the smaller splittings are due to the coupling with the second β-hydrogen as well as the hydrogens in position 3,5 of the aromatic ring.6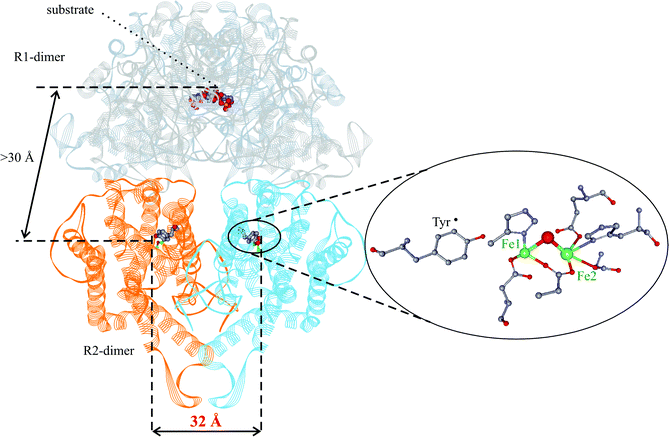 | ||
| Fig. 1 Structure of E. coli RNR modeled from the two separately solved structures of R1 and R2 dimers.4 The tyrosyl radical and the diiron(III) center are shown on an enlarged scale (right). | ||
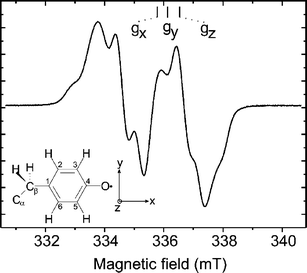 | ||
| Fig. 2 cw-EPR (X-band) of the tyrosyl radical of mouse RNR recorded at T = 40 K; νmw = 9.4314 GHz; microwave power Pmw 0.2 mW; scan time 84 s. The structure of the tyrosyl radical is shown with numbering scheme together with the molecular axis system. | ||
The R2 protein is activated by incubating the apo-protein in the presence of iron(II) and oxygen. In this process the tyrosyl radical is generated. The in vitro reconstituted RNR contains up to 0.8 tyrosyl radicals per monomer.7 Therefore it can be assumed that two radicals are simultaneously present in the R2 homodimer ββ.
Pulse electron–electron double resonance (PELDOR)8–11 proved to be suitable to determine the distance between two paramagnetic centers when the distance is in the range from about 1.5 to 8.0 nm. The method can be applied to the R2 dimer when both monomers carry a tyrosyl radical. Bennati et al.12 have successfully employed PELDOR on R2 from Escherichia coli RNR, a well characterized system for which an estimate of the distance between the two tyrosine residues already existed from the crystal structure of the dimeric ‘met form’, which contains the μ-oxo-diferric center but the tyrosine is not in the radical form. As the distance determined by four-pulse PELDOR (3.31 nm) is in good agreement with the distance measured from the crystal structure (3.26 nm),13 the authors proved the effectiveness of using PELDOR for distance determinations in RNR proteins. The method can therefore be employed to gain such information also on less characterized proteins, for which a crystal structure is lacking.
For mouse R2 the crystal structure has also been published,14 but the studies have been performed at pH = 4.7 far from the physiological condition (pH = 7.5) and only the crystal structure of a monomeric form with only one iron ion per monomer has been obtained. Strand et al.15 have recently improved the crystal structure studies by incubating crystals at pH = 6.0 and incorporating two iron ions per subunit in both the reduced and the oxidized form but the overall structure is still identical to that at pH 4.7. Only the structure of the monomeric form has been obtained and no tyrosyl radicals are present in these crystals. Therefore it is important to obtain more information about this protein, in particular in the active state and under physiological conditions.
In the present work, PELDOR has been employed, for the first time, to study the intact enzyme R2 of mouse RNR under physiological conditions. It is demonstrated that the PELDOR study provides fundamental information such as clear evidence of the dimeric structure of the enzyme in solution and the accurate distance between the two Y˙ radicals in the active dimeric protein.
2. Materials and methods
2.1 Sample preparation
Mouse R2 ribonucleotide reductase protein (mouse-R2) was prepared as described earlier.7 Activated protein was obtained by incubating the apo-R2 protein in aerobic Tris-buffer (50 mM Tris, 100 mM KCl, pH = 7.5) with an eight-fold molar excess of Fe(II), i.e. 8 Fe/ββ. An aerobic (NH4)2Fe(SO4)2 solution was prepared in 3 mM H2SO4 (pH = 2.3) and mixed with the protein solution in a v/v ratio of 1∶10 (mouse-R2-h). Thus, the (NH4)2Fe(SO4)2 concentration was 80-fold higher than the R2 concentration. This mixing ensures that the pH of 7.5 is not altered. A sample was also prepared in deuterated buffer (mouse-R2-d). To the reconstituted protein 5% (v/v) of glycerol was added, in both mouse-R2-h and mouse-R2-d; without glycerol the relaxation time is too fast for measuring PELDOR, at higher glycerol concentrations, e.g. 40%, at which a perfect glass is formed upon freezing, the electron T1 relaxation time becomes so slow that the experiments get cumbersome. The final concentration of the protein was 200 μM, with about 1.6 radicals per dimer. The radical concentration of Y˙ was determined by comparing the integrated EPR intensity to that of a calibrated copper standard.2.2 PELDOR measurements
PELDOR experiments have been performed on a Bruker ELEXSYS E580 SuperXFT spectrometer equipped with a dielectric ring resonator (Bruker ER4118 SPT) for the X-band experiments and a Bruker ELEXSYS E580 SuperQFT for Q-band equipped with a home-built resonator. For the Q-band PELDOR experiments a brass cylindrical TE011 resonator was used (id 11.5 mm). The resonance frequency of the cavity was adjustable by a movable plunger over a range of about 2 GHz. The microwaves were coupled through an iris hole in the side of the cylinder, the diameter of which was chosen to slightly overcouple the resonator (with loaded sample). The coupling could be adjusted by a sliding short in the coupling waveguide. The resonator allowed a sample tube of 3 mm od.The sequences used for the PELDOR experiments is given in Fig. 3. All spectra have been recorded at T = 6 K using an Oxford CF935 cryostat. The three-pulse and four-pulse PELDOR are equivalent techniques providing the same information except that four-pulse has the advantage to be dead time free16 but requires a longer evolution time to record a spectrum with the same number of modulations as obtained in a three-pulse experiment. Therefore the three-pulse technique is preferred in the case of weak signals and is used for a preliminary characterization of the PELDOR effect. The four-pulse technique is preferred when a distribution of distances is expected. In this case, the first part of the PELDOR trace is particularly important.16
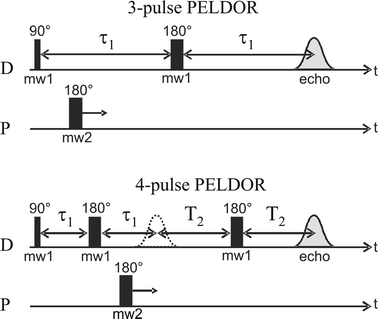 | ||
| Fig. 3 The three- and four-pulse PELDOR sequences used in this work. The detection (D) and pumping (P) pulse lengths employed for the PELDOR experiments are as follows. X-band three-pulse: (D) 16 ns–32 ns, (P) 32 ns. X-band four-pulse: (D) 16 ns–32 ns–32 ns, (P) 32 ns. Q-band three-pulse: (D) 148 ns–300 ns, (P) 60 ns. | ||
Q-band PELDOR experiments have been performed at different positions of the EPR spectrum spaced by 1.0 mT (28 MHz). The lower signal-to-noise ratio of PELDOR spectra at Q-band compared with X-band is due to the lower microwave power (about 0.5 W). This is sufficient to generate π-pulses of 30 ns (with 30 MHz bandwidth) corresponding to a B1-field of about 0.3 mT. For both the X-band and Q-band PELDOR experiments an offset between the two microwave frequencies of 60 MHz was used. In the Q-band experiment, however, the resonator bandwidth did not allow both frequencies to be excited with the same efficiency. This is in contrast to the X-band experiment where the dielectric resonator in combination with the TWT amplifier (Applied System Engineering, Inc.) allows for a bandwidth of 100 MHz. Therefore, the signal-to-noise ratio of the Q-band PELDOR spectra is substantially lower than that of the X-band spectra.
2.3 Spectral analysis
The PELDOR spectra have been analyzed using the program DeerAnalysis 2004 developed by Jeschke.17 The spectra have been fitted using the model-free distance-domain Tikhonov regularization for narrow distributions that provides the distance distribution.18The frequency of the PELDOR modulation is inversely proportional to the cube of the distance between the radicals:
2.4 Relaxation times T1 and Tm measurements
As described above, glycerol has been added to the sample to allow performing PELDOR experiments. To optimize the effect of the glycerol content on the electron spin relaxation times of the tyrosyl radical for the PELDOR experiment, both the longitudinal relaxation time T1 and the phase-memory time Tm have been measured on the mouse-R2-h sample at 6 K, T1 has been measured also at 30 K.T 1 was obtained from saturation recovery experiments employing a train of eight soft pulses to saturate the Hahn echo; Tm was measured by the Carr–Purcell–Meiboom–Gill sequence8.
3. Results and discussion
In Fig. 4A the echo-detected X-band EPR spectrum of the sample mouse-R2-d is shown, the arrows indicate the position of the detection (D) and pump frequency (P), the difference between the two frequencies was 60 MHz. The four-pulse PELDOR X-band spectrum recorded on the same sample (Fig. 4B) showed deep and slowly decaying modulations that were analyzed with the DeerAnalysis 2004 program to obtain the best fit and an accurate distance distribution between the two radicals (Fig. 4C). The analysis of the distance distribution (Fig. 4C) shows a narrow peak centered at 3.25 ± 0.05 nm. The value is similar to the one previously reported for E. coli R2 (3.31 nm).12 Note that 3.26 nm was obtained from the X-ray crystal structure of E. coli R2.13 The value represents the distance between the centers of gravity of the spin density of the two radicals. For an estimation of the center of gravity of spin density (ξ) of the tyrosyl radical a p-ethylphenoxyl radical model system (see sketch in Fig. 2, Cα is CH3) with an H-bond to a H2O (syn to Cα) was used.6 Geometry optimization and atomic spin populations were obtained as described earlier.19 The dihedral angle Cα–Cβ–C1–C2 was fixed to its experimental estimate of 33°. The following spin densities above 1% were obtained: C1: +0.371, C2: −0.133, C3: +0.279, C4: −0.036, C5: +0.263, C6: −0.125, O: +0.385, Hβ: +0.025, H(C3): −0.014, and H(C5): −0.013 (sum 1.002). The ξ is defined as the sum over all products of spin density σ and distance vector![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) ,
, ![[small xi, Greek, vector]](https://www.rsc.org/images/entities/i_char_e1cd.gif) = ∑σ·
= ∑σ·![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) from a common zero point which was chosen to be the center of the tyrosine ring. All directions were taken as collinear with the g-tensor (Fig. 2). Allowing an uncertainty of 0.01 nm, well below 0.05 nm from the PELDOR evaluation, all z-components can be neglected and y-components cancel out each other. The calculation showed that the ξ is located on the C1–C4–O axes 0.05 nm distant from C4 towards the center of the tyrosine ring and 0.24 nm away from C1.
from a common zero point which was chosen to be the center of the tyrosine ring. All directions were taken as collinear with the g-tensor (Fig. 2). Allowing an uncertainty of 0.01 nm, well below 0.05 nm from the PELDOR evaluation, all z-components can be neglected and y-components cancel out each other. The calculation showed that the ξ is located on the C1–C4–O axes 0.05 nm distant from C4 towards the center of the tyrosine ring and 0.24 nm away from C1.
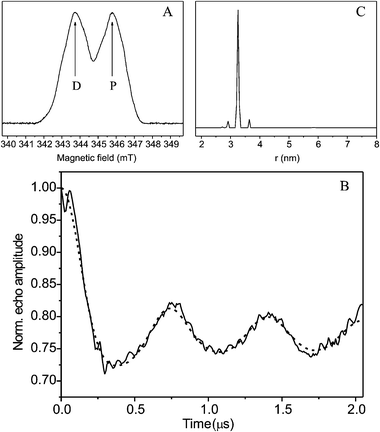 | ||
| Fig. 4 (A) Echo detected X-band (9.686 GHz) EPR spectrum of mouse-R2-d; the arrows indicate the frequencies of the detection (D) and pumping (P) pulses; (B) Four-pulse X-band PELDOR of mouse-R2-d: experimental (solid line) and simulated (dashed line) spectrum computed with the DeerAnalysis 2004 program.17 (C) Analysis of distance distribution of the two Y˙ radicals in the dimer obtained with the DeerAnalysis 2004 program.17 For further experimental conditions see both Fig. 3 and Fig. 5. | ||
The hard pulses used in the four-pulse PELDOR sequence can give rise to nuclear modulation effects from deuterium that can interfere with the PELDOR analysis. To rule out such effects three-pulse PELDOR experiments at X-band have been performed on the samples mouse-R2-d (Fig. 5b) and mouse-R2-h (Fig. 5c). Compared with the four-pulse spectra (Fig. 5a) the three-pulse spectra (Fig. 5b,c) show no visible differences. By comparing the spectra of R2 in deuterated (Fig. 5b) and non-deuterated (Fig. 5c) buffer one can rule out that the observed modulation is due to modulations of the electron-deuterium dipolar interaction. Thus, it originates indeed from the dipole–dipole interaction between the two electron spins of the tyrosyl radicals.
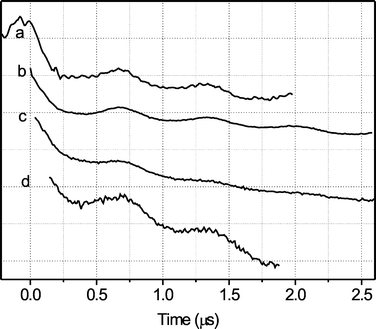 | ||
| Fig. 5 PELDOR spectra (T = 6 K; pulse sequence is described in Fig. 3; number of scans: a = 56, b = 206, c = 25, d = 396): (a) four-pulse X-band of mouse-R2-d; (b) three-pulse X-band of mouse-R2-d; (c) three-pulse X-band of mouse-R2-h; (d) three-pulse Q-band of mouse-R2-d, obtained at g = 2.0030 | ||
Since the protein has not yet been crystallized in the dimeric form, it is important to establish the relative orientation of the two radicals to each other. This can be obtained by performing orientation-selective PELDOR experiments. However, the g-components along the principal axes strongly overlap in the EPR spectrum at X-band (Fig. 2). At Q-band the situation is somewhat more favorable. However, the g-anisotropy in frequency units (60 MHz) is still close to the bandwidth of the exciting PELDOR pulses (30 MHz) thus limiting the orientational selection. In order to test the efficiency of orientation selection, PELDOR experiments have been performed at Q-band (34 GHz) on different magnetic field positions in steps of 1.0 mT. Due to the weaker signal at Q-band compared to X-band, a three-pulse PELDOR has been chosen. However, no evident differences could be observed, neither between the spectra recorded at different magnetic fields nor between spectra recorded at X-band and Q-band. A representative spectrum is shown in Fig. 5d. Obviously, orientation selection in PELDOR experiments on Y˙ requires microwave frequencies significantly higher than Q-band (34 GHz).‡
To be able to perform PELDOR experiments, it has been necessary to add glycerol to the samples. The effect of addition of glycerol on the electron relaxation times has been investigated by measuring both the electron spin lattice relaxation, T1, and phase memory time, Tm, at different concentrations of glycerol (spectra not shown). Both relaxation times, measured at 6 K for mouse-R2-h, slowed down when the glycerol concentration was increased. For 0, 5, 10, and 40% glycerol T1 was measured to 3, 16, 24, and 177 ms, respectively, whereas for Tm 2, 5, 5, and 8 μs was obtained. Both relaxation times showed a biexponential decay, only the slower component, that is the relevant one to optimize PELDOR experiments, is listed. Note that for T1 a more than 50-fold change was detected. At 40% glycerol a perfect glass is obtained upon freezing which is not observed at lower concentrations. The change of T1 upon the glycerol content of the sample is vanishingly small for elevated temperatures (T ≥ 30 K). To clarify this behavior of the relaxation properties further experiments are planned.
4. Conclusions
Pulse electron–electron double resonance has been applied to measure the distance between the tyrosyl radicals in the R2 subunit of mouse ribonucleotide reductase. The PELDOR results clearly show that the protein is in a dimeric form and the distance between the two tyrosyl radicals present in the dimer halves is 3.25 ± 0.05 nm. This provides important structural information on a mammalian enzyme that is closely related to human RNR, for which no X-ray crystallographic structure exists so far for the native (active) form. The near identity of the distance between the two tyrosyls in mouse R2 and in E. coli R2, strongly suggests that mouse R2 forms a dimer that closely resembles that of E. coli R2. To obtain the relative orientation between the two Y˙ radicals further PELDOR investigations at higher fields (≥100 GHz) are planned. The effect of glycerol on the relaxation properties of the tyrosyl radical will be further investigated.Acknowledgements
The authors are grateful to Dr Marina Bennati (Frankfurt/Main, Germany) for her suggestions to improve the PELDOR measurements and Dr Sebastian Sinnecker (Mülheim/Ruhr, Germany) for providing the spin density distribution of the tyrosyl radical in model. We also thank Prof. Lars Thelander (Umeå, Sweden) for supplying us with mouse R2 protein over-expressing E. coli cells. This research was supported by Max Planck Society and DFG (Schm-1676/1-1).References
- H. Eklund, U. Uhlin, M. Färnegårdh, D. T. Logan and P. Nordlund, Prog. Biophys. Mol. Biol., 2001, 77, 177–268 CrossRef CAS.
- M. Kolberg, K. R. Strand, P. Graff and K. K. Andersson, Biochim. Biophys. Acta, 2004, 1699, 1–34 CAS.
- P. Reichard, Arch. Biochem. Biophys., 2002, 397, 149–155 CrossRef CAS.
- U. Uhlin and H. Eklund, Nature, 1994, 370, 533–539 CAS.
- F. Lendzian, Biochim. Biophys. Acta, 2005, 1707, 67–90 CAS.
- P. P. Schmidt, K. K. Andersson, A.-L. Barra, L. Thelander and A. Gräslund, J. Biol. Chem., 1996, 271, 23615–23618 CrossRef CAS.
- P. P. Schmidt, U. Rova, B. Katterle, L. Thelander and A. Gräslund, J. Biol. Chem., 1998, 273, 21463–21472 CrossRef CAS.
- A. Schweiger and G. Jeschke, Principles of Pulse Electron Paramagnetic Resonance, Oxford University Press, Oxford, 2001 Search PubMed.
- A. D. Milov, A. B. Ponomarev and Y. D. Tsvetkov, Chem. Phys. Lett., 1984, 110, 67–72 CrossRef CAS.
- A. D. Milov, D. A. Erilov, E. S. Salnikov, Yu. D. Tsvetkov, F. Formaggio, C. Toniolo and J. Raap, Phys. Chem. Chem. Phys., 2005, 8, 1794–1799 RSC.
- A. D. Milov, Yu. D. Tsvetkov, F. Formaggio, S. Oancea, C. Toniolo and J. Raap, Phys. Chem. Chem. Phys., 2004, 13, 3596–3603 RSC.
- M. Bennati, A. Weber, J. Antonic, D. L. Perlstein, J. Robblee and J. Stubbe, J. Am. Chem. Soc., 2003, 125, 14988–14989 CrossRef CAS.
- M. Högbom, M. Galander, M. Andersson, M. Kolberg, W. Hofbauer, G. Lassmann, P. Nordlund and F. Lendzian, Proc. Natl. Acad. Sci. USA, 2003, 100, 3209–3214 CrossRef.
- B. Kauppi, B. B. Nielsen, S. Ramaswamy, I. K. Larsen, M. Thelander, L. Thelander and H. Eklund, J. Mol. Biol., 1996, 262, 706–720 CrossRef CAS.
- K. R. Strand, S. Karlsen, M. Kolberg, Å. K. Røhr, C. H. Görbitz and K. K. Andersson, J. Biol. Chem., 2004, 279, 46794–46801 CrossRef CAS.
- R. E. Martin, M. Pannier, F. Diederich, V. Gramlich, M. Hubrich and H. W. Spiess, Angew. Chem., Int. Ed., 1998, 37, 3833–3837.
- G. Jeschke, DeerAnalysis, Max Planck Institute for Polymer Research, Mainz, Germany, 2004, http://www.mpip-mainz.mpg.de/∼jeschke/distance.html Search PubMed.
- G. Jeschke, G. Panek, A. Godt, A. Bender and H. Paulsen, Appl. Magn. Reson., 2004, 26, 223–244 CrossRef CAS.
- S. Sinnecker, E. Reijerse, F. Neese and W. Lubitz, J. Am. Chem. Soc., 2004, 126, 3280–3290 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. 3 and 4 in colour. See DOI: 10.1039/b513950c |
| ‡ A pilot study at W-band (94 GHz) has been performed: the low signal-to-noise ratio of the PELDOR experiment did not allow us to successfully analyze the spectra. |
| This journal is © the Owner Societies 2006 |