Improving success rate of hydrogen-bond driven synthesis of co-crystals†
Christer B.
Aakeröy
*,
John
Desper
and
Meg E.
Fasulo
Department of Chemistry, Kansas State University, Manhattan, KS 66506, USA. E-mail: aakeroy@ksu.edu
First published on 19th July 2006
Abstract
Increasing the negative charge of a hydrogen-bond acceptor using electron-donating substituents offers a significant improvement in hydrogen-bond driven synthesis of carboxylic acid⋯N-heterocycle co-crystals.
The ability to construct heteromeric multi-component molecular architectures with desirable connectivities and metrics is a highly desirable goal for a broad spectrum of supramolecular synthetic chemists.1 By developing and refining the fundamental aspects of the assembly of such systems it may also be possible to translate intermolecular communication into blueprints for biological mimics or for materials design. One way this can be accomplished is to map out the behavior of intermolecular interactions and the structural preference of common chemical functionalities in a deliberate manner. Establishing the balance between, and importance of, a variety of intermolecular interactions can be achieved through systematic co-crystallization experiments, and may lead to reliable synthetic protocols for supramolecular synthesis. In order to form a co-crystal between an N-heterocycle and a carboxylic acid, the basic assumption is that the two components must be able to engage in a heteromeric intermolecular interaction that can compete successfully with the commonly occurring acid⋯acid homomeric dimer, otherwise the two solutes will appear as separate homomeric solids (an example of a typical recrystallization).2 In this study, we set out to determine if the strength of a single O–H⋯N hydrogen-bond interaction can be directly related to the relative success with which a desired family of co-crystals can be synthesized.
1,4-Bis[(imidazol-1-yl)methyl]benzene, 1,4-bis[(benzimidazol-1-yl)methyl]benzene, 4,4′-bipy and related N-heterocycles are known to form co-crystals with carboxylic acids, driven by O–H⋯N hydrogen bonds.3 The negative electrostatic potential of the nitrogen atom in these compounds represents an attractive and competitive binding site for an approaching carboxylic acid. The analogous pyrazole and 3,5-dimethylpyrazole are both significantly less basic than py or imidazole moieties, but there is also an appreciable difference between the two N-heterocycles; 3,5-dimethylpyrazole is more basic than pyrazole due to the electronic influence of the two electron-donating substituents. If the strength of an O–H⋯N hydrogen bond were a direct measure of how easy/difficult it is to form co-crystals with a range of hydrogen-bond donors, one would expect a greater success rate with the more basic 3,5-dimethylpyrazole as an acceptor than with pyrazole.4
In order to test this hypothesis we synthesized two ditopic symmetric ligands, 1,4-bis[(pyrazol-1-yl)methyl]benzene, 1 and 1,4-bis[(3,5-dimethylpyrazol-1-yl)methyl]benzene, 2,5 (calculated molecular electrostatic potentials and pKa values are shown in Scheme 1).6,7 In addition, 1 and 2 provide a good test system because solubility is similar for the two, and the addition of the methyl groups in 2 does not hinder access to the hydrogen-bonding sites.
 | ||
| Scheme 1 | ||
Each compound, 1–2, was combined in a 2 ∶ 1 acid–ligand ratio with thirty different carboxylic acids. The reactants were dissolved together in ethanol and the resulting solution was slowly evaporated to dryness. We did not want to bias our observations and results by only considering single-crystal structure-determination as our analytical tool, and consequently all solid products were characterized by infrared spectroscopy to determine if a co-crystal had formed. The presence of two broad bands at ca. 2500 cm−1 and 1900 cm−1, characteristic of an O–H⋯N (acid⋯N-heterocycle) hydrogen-bond interaction, was viewed as evidence for co-crystal formation, whereas the absence of such bands was interpreted as a lack of co-crystal formation, Table 1.8
| Acid | 1 | 2 |
|---|---|---|
| Benzoic acid | — | — |
| 4-Chlorobenzoic acid | — | — |
| 3-Chlorobenzoic acid | — | 2456, 1911 |
| 2-Chlorobenzoic acid | — | — |
| 4-Cyanobenzoic acid | 2528, 1912 | 2428, 1931 |
| 3-Cyanobenzoic acid | — | 2427, 1931 |
| 2-Chloro-6-fluorobenzoic acid | 2484, 1895 | 2407, 1936 |
| 2-Chloro-4-fluorobenzoic acid | — | 2428, 1906 |
| 2,6-Dichlorobenzoic acid | 2469, 1906 | 2387, 1916 |
| 2,5-Dichlorobenzoic acid | 2484, 1931 | 2413, 1931 |
| 2,4-Dichlorobenzoic acid | — | 2397, 1916 |
| 3,4-Dichlorobenzoic acid | — | 2571, 1931 |
| 2,6-Difluorobenzoic acid | 2479, 1900 | — |
| 2,4-Difluorobenzoic acid | — | — |
| 3,5-Dihydroxybenzoic acid | — | — |
| 2,4-Dimethoxybenzoic acid | — | — |
| 4-Dimethylaminobenzoic acid | — | 2469, 1931 |
| 3-Dimethylaminobenzoic acid | — | 2469, 1895 |
| 3,5-Dinitrobenzoic acid | 2407, 1906 | 2510, 1906 |
| 4-Fluorobenzoic acid | — | — |
| 2-Fluorobenzoic acid | — | 2469, 1916 |
| 3-Hydroxybenzoic acid | — | 2597, 1921 |
| 4-OH-3-methoxycinnamic acid | — | — |
| 4-Iodobenzoic acid | 2568, 1905 | 2561, 1931 |
| 3-Iodobenzoic acid | — | — |
| 1-Naphthoic acid | — | 2515, 1946 |
| 4-Nitrobenzoic acid | 2510, 1916 | 2433, 1947 |
| 3-Nitrobenzoic acid | 2459, 1898 | 2423, 1911 |
| Pentafluorobenzoic acid | 2428, 1916 | 2392, 1936 |
| Pentamethylbenzoic acid | 2556, 1900 | 2500, 1987 |
| = 11/30 (37%) | = 20/30 (67%) |
Crystals suitable for single-crystal structure determination of three representative compounds were subsequently grown by slow evaporation from ethanol in order to ensure that the intermolecular interactions and stoichiometries were present as expected. The crystal structure determination of the product, 1a, obtained from the reaction between 1 and 3,5-dinitrobenzoic acid shows that the expected 1 ∶ 2 co-crystal was formed with the pyrzole-based ligand located about an inversion center.9 The assembly is driven by the heteromeric O–H⋯N hydrogen bond between the O–H group on the carboxylic acid and the pyrazol-1-yl nitrogen atom (O31⋯N12, 2.5786(14) Å), Fig. 1. There has been no proton transfer between the acid and the base, and there are no auxiliary C–H⋯O interactions between the carbonyl oxygen (O32) and a C–H moiety of the adjacent pyrazole ring.
![1 ∶ 2 binary co-crystal of 1,4-bis[(pyrazol-1-yl)methyl]benzene 1 and 3,5-dinitrobenzoic acid.](/image/article/2006/CE/b609709j/b609709j-f1.gif) | ||
| Fig. 1 1 ∶ 2 binary co-crystal of 1,4-bis[(pyrazol-1-yl)methyl]benzene 1 and 3,5-dinitrobenzoic acid. | ||
The crystal structure determination of the product, 2a, from the reaction between 2 and 2,6-dichlorobenzoic acid shows a co-crystal assembled via an O–H⋯N hydrogen interaction between the carboxylic acid and the available nitrogen atoms on 2 (O31⋯N22, 2.598(3) Å), Fig. 2.10 Again, the bis-heterocycle is located about an inversion center. There are auxiliary C–H⋯O interactions between the carbonyl oxygen and a C–H moiety of the methylene bridge (O32⋯C17, 3.427 Å).
 | ||
| Fig. 2 1 ∶ 2 binary co-crystal of 2 and 2,6-dichlorobenzoic acid. | ||
2b is a co-crystal composed of 2 and pentamethylbenzoic acid in the expected 1 ∶ 2 ratio.11 In this case there are two inequivalent supermolecules constructed through heteromeric O–H⋯N hydrogen bonds (O81⋯N32, 2.667(3) Å; O61⋯N12, 2.675(3) Å), Fig. 3. Both heterocyclic compounds are located about inversion centers. There are no auxiliary interactions between the carbonyl oxygen atoms (O62, O82) and the C–H moieties of the pyrazole rings.
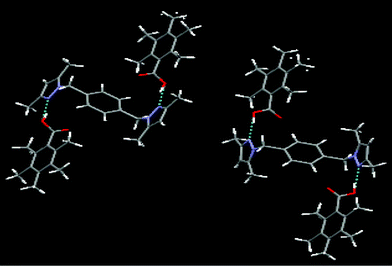 | ||
| Fig. 3 1 ∶ 2 binary co-crystal of 2 and pentamethylbenzoic acid. | ||
Based upon a systematic analysis of the spectroscopic data (supported by three single-crystal structure determinations) it is clear that 3,5-dimethylpyrazole produces many more co-crystals than the corresponding pyrazole ligand, 20/30 (67) vs. 11/30 (37%).
The covalent modifications that distinguish 1 from 2 have increased the magnitude of the negative electrostatic potential on the nitrogen atom in the latter compound, which is also reflected in the basicity of 2, Scheme 1.
It is quite possible that additional co-crystals can be formed with either 1 or 2 and acids listed in Table 1 if a variety of solvents and/or different reaction conditions are employed, however, it is unlikely that the relative success rate of the two ligands with respect to each other will change significantly. Since 1 and 2 display many chemical similarities, it is reasonable to deduce, based upon the results obtained in this study, that the superiority of 2 as a co-crystallizing agent is directly related to the increased strength of the resulting O–H⋯N hydrogen bond that it can form with a range of carboxylic acids. These results complement previous findings12 that demonstrate that the relative acidity/positive electrostatic potential of oxime moieties is directly related to supramolecular yield of oxime⋯N-heterocycle co-crystals. Consequently, simple changes in molecular structure can alter hydrogen-bonding capability in a controlled manner that, in turn, provides a handle for fine-tuning supramolecular reactivity. It is reasonable to assume that this observation can be translated into a tool that can facilitate practical hydrogen-bond based supramolecular synthesis of co-crystals involving a wide range of components. Furthermore, by applying these ideas to more complicated supramolecular building blocks with inequivalent hydrogen-bond acceptor sites, it may be easier to construct predictable, multi-component supramolecular systems with greater structural and chemical complexity.
We are grateful for financial support from NSF (CHE-0316479), Howard Hughes Medical Institute, and Terry C. Johnson Center for Basic Cancer Research.
Notes and references
- C. B. Aakeröy and D. J. Salmon, CrystEngComm, 2005, 7, 439 RSC; L. Sreenivas Reddy, N. Jagadeesh Babu and Ashwini Nangia, Chem. Commun., 2006, 1369 RSC; A. V. Trask, J. van de Streek, S. W. D. Motherwell and W. Jones, Cryst. Growth Des., 2005, 5, 2233 CrossRef CAS; Ö. Almarsson and M. J. Zaworotko, Chem. Commun., 2004, 1889 RSC.
- C. B. Aakeröy, J. Desper and B. A. Helfrich, CrystEngComm, 2004, 6, 19 RSC.
- C. B. Aakeröy, J. Desper, B. Leonard and J. F. Urbina, CrystEngComm, 2005, 5, 865 Search PubMed; C. B. Aakeröy, I. Hussain and J. Desper, Cryst. Growth Des., 2006, 6, 474 CrossRef; B. R. Bhogala, S. Basavoju and A. Nangia, Cryst. Growth Des., 2005, 5, 1683 CrossRef CAS; S. M. Curtis, N. Le, F. W. Fowler and J. W. Lauher, Cryst. Growth Des., 2005, 5, 2313 CrossRef CAS; B. K. Saha, A. Nangia and M. Jaskolski, CrystEngComm, 2005, 7, 355 RSC; P. Vishweshwar, J. A. McMahon, M. L. Peterson, M. B. Hickey, T. R. Shattock and M. J. Zaworotko, Chem. Commun., 2005, 4601 RSC; M. W. Hosseini, CrystEngComm, 2004, 6, 318 RSC; G. R. Desiraju, Acc. Chem. Res., 2002, 35, 565 CrossRef CAS; C. B. Aakeröy, J. Desper and B. M. T. Scott, Chem. Commun., 2006, 1445 RSC; C. B. Aakeröy, J. Desper, D. J. Salmon and M. M. Smith, Cryst. Growth Des., 2006, 6, 1033 CrossRef.
- M. C. Etter, Acc. Chem. Res., 1990, 23, 120 CrossRef CAS; M. C. Etter, J. Phys. Chem., 1991, 95, 4601 CrossRef CAS.
- C. M. Hartshorn and P. J. Steel, Aust. J. Chem., 1995, 48, 1587 CAS.
- The two ligands shown in Scheme 1 were constructed using Spartan '04 (Wavefunction, Inc. Irvine, CA). Their molecular geometries were optimized using AM1, and the maxima and minima in the molecular electrostatic potential surface (0.002 e au−1 isosurface) were determined using a positive point charge in vacuum as the probe.
- pKa values were obtained through calculations of the conjugate acids. The calculations were carried out using ACD/Solaris version 4.76, Advanced Chemistry Development, Inc., Toronto, ON, Canada, 1994–2005, www.acdlabs.com Search PubMed.
- A carboxylic acid and 1 (or 2) could in theory form a co-crystal without engaging in a heteromeric O–H⋯N hydrogen bond but it is highly unlikely.
- Crystallographic data for 1a, 1,4-bis[(pyrazol-1-yl)methyl]benzene, 3,5-dinitrobenzoic acid (1 ∶ 2): C28H22N8O12, M = 662.54, monoclinic, space group P2(1)/c, a = 12.526(3) Å, b = 10.179(2) Å, c = 12.332(3) Å, α = 90°, β = 115.038(4)°, γ = 90°, V = 1424.6(5) Å3, Z = 2, Dc = 1.545 g cm−3, μ(Mo-Kα) = 0.124 mm−1, crystal size 0.10 × 0.22 × 0.34 mm. Data were collected at 100 K on a Bruker SMART 1000 diffractometer using Mo-Kα radiation. A total of 14640 reflections (2.69° < θ < 30.10°) were processed of which 4156 were unique and significant with I > 2σ(I). Final residuals for I > 2σ(I) were R1 = 0.0453, and wR2 = 0.1194 (GOF = 1.055).
- Crystallographic data for 2a, 1,4-bis[(3,5-dimethylpyrazol-1-yl)methyl]benzene, 2,6-dichlorobenzoic acid (1 ∶ 2): C32H30 Cl4N4O4, M
= 676.40, triclinic, space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a
= 7.5783(8)
Å, b
= 7.9010(9)
Å, c
= 14.1442(13)
Å, α
= 103.784(7)°, β
= 101.429(7)°, γ
= 90.509(6)°, V
= 804.80(15)
Å3, Z
= 1, Dc
= 1.396 g cm−3, μ(Mo-Kα)
= 0.411 mm−1, crystal size 0.40 × 0.40 × 0.15 mm. Data were collected at 100 K on a Bruker SMART 1000 diffractometer using Mo-Kα radiation. A total of 6024 reflections (1.51° < θ < 27.89°) were processed of which 3580 were unique and significant with I > 2σ(I). Final residuals for I > 2σ(I) were R1
= 0.0666, and wR2
= 0.1956 (GOF = 1.357).
, a
= 7.5783(8)
Å, b
= 7.9010(9)
Å, c
= 14.1442(13)
Å, α
= 103.784(7)°, β
= 101.429(7)°, γ
= 90.509(6)°, V
= 804.80(15)
Å3, Z
= 1, Dc
= 1.396 g cm−3, μ(Mo-Kα)
= 0.411 mm−1, crystal size 0.40 × 0.40 × 0.15 mm. Data were collected at 100 K on a Bruker SMART 1000 diffractometer using Mo-Kα radiation. A total of 6024 reflections (1.51° < θ < 27.89°) were processed of which 3580 were unique and significant with I > 2σ(I). Final residuals for I > 2σ(I) were R1
= 0.0666, and wR2
= 0.1956 (GOF = 1.357). - Crystallographic data for 2b, 1,4-bis[(3,5-dimethylpyrazol-1-yl)methyl]benzene, pentamethylbenzoic acid (1 ∶ 2): C42H54N4O4, M
= 678.89, triclinic, space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a
= 8.8093(6)
Å, b
= 14.7493(10)
Å, c
= 15.4238(12)
Å, α
= 67.222(4)°, β
= 82.777(4)°, γ
= 86.232(4)°, V
= 1832.8(2)
Å3, Z
= 2, Dc
= 1.230 g cm−3, μ(Mo-Kα)
= 0.079 mm−1, crystal size 0.30 × 0.25 × 0.15 mm. Data were collected at 100 K on a Bruker SMART 1000 diffractometer using Mo-Kα radiation. A total of 13688 reflections (1.44° < θ < 27.49°) were processed of which 3580 were unique and significant with I > 2σ(I). Final residuals for I > 2σ(I) were R1
= 0.0798, and wR2
= 0.2154 (GOF = 1.231). CCDC reference numbers 607722–607724. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b609709j.
, a
= 8.8093(6)
Å, b
= 14.7493(10)
Å, c
= 15.4238(12)
Å, α
= 67.222(4)°, β
= 82.777(4)°, γ
= 86.232(4)°, V
= 1832.8(2)
Å3, Z
= 2, Dc
= 1.230 g cm−3, μ(Mo-Kα)
= 0.079 mm−1, crystal size 0.30 × 0.25 × 0.15 mm. Data were collected at 100 K on a Bruker SMART 1000 diffractometer using Mo-Kα radiation. A total of 13688 reflections (1.44° < θ < 27.49°) were processed of which 3580 were unique and significant with I > 2σ(I). Final residuals for I > 2σ(I) were R1
= 0.0798, and wR2
= 0.2154 (GOF = 1.231). CCDC reference numbers 607722–607724. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b609709j. - C. B. Aakeröy, D. J. Salmon, M. M. Smith and J. Desper, Cryst. Growth Des., 2006, 6, 1033 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of supramolecular reagents. See DOI: 10.1039/b609709j |
| This journal is © The Royal Society of Chemistry 2006 |
