A diverse suite of spiroacetals, including a novel branched representative, is released by female Bactrocera tryoni (Queensland fruit fly)†
Yvonne K.
Booth
a,
Brett D.
Schwartz
a,
Mary T.
Fletcher
ab,
Lynette K.
Lambert
c,
William
Kitching
a and
James J. De
Voss
*a
aChemistry, School of Molecular and Microbial Sciences, University of Queensland, St. Lucia, Brisbane, Australia 4072. E-mail: j.devoss@uq.edu.au; Fax: +61 7 3365 4299; Tel: +61 7 3365 3825
bDepartment of Primary Industries, Yeerongpilly, Brisbane, Australia 4105
cCentre for Magnetic Resonance, University of Queensland, St. Lucia, Brisbane, Australia 4072
First published on 6th September 2006
Abstract
A remarkably diverse suite of spiroacetals including a novel member of the rare, branched chain class has been identified in the glandular secretions of Bactrocera tryoni, the most destructive horticultural pest in Australia.
Bactrocera tryoni (Frogatt) (Queensland fruit fly) is the most damaging horticultural pest in Australia, having in excess of 200 host plants and being found all along the eastern coast. The annual cost to the horticultural industry is estimated to be AUD$500 million, through lost production, population monitoring and control programs.1 In pursuit of more selective and environmentally benign control measures, recent interest has focused on the potential use of pheromone based methods, which have become important for controlling Lepidopteran and other species.2 Pheromones are often multicomponent and synergistic in nature, with their specificity and biological activity being determined in part by the chirality, geometry, structure, and ratios of the components released by the insects. These factors are emphasised in the present study of B. tryoni.
Sexually excited B. tryoni males secrete an oily blend of six amides, which functions as a short range attractant and invokes responses in mature females.3 In contrast, however, the chemistry of females has not been formally reported.4 We now describe comprehensive analyses of volatile emissions and pentane abdominal extracts of female B. tryoni. More than a dozen spiroacetal stereoisomers, including a novel member of the rare branched chain spiroacetal class, have been identified.
Analysis of the volatile emissions from B. tryoni females using SPME-GCMS revealed that the major components (Fig. 1) were N-(3-methylbutyl)propanamide (1) and the spiroacetal (E,E)-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane‡ (2), which has been reported from a wide variety of insect species, including the closely related fruit fly B. cucumis.5N-(3-methylbutyl)acetamide (3) and two additional spiroacetals, viz. (E,E)-2-ethyl-8-methyl-1,7-dioxaspiro[5.5]undecane (4) and (E,E)-2-propyl-8-methyl-1,7-dioxaspiro[5.5]undecane (5), were also found at low levels. Spiroacetals 4 and 5 have both previously been reported from B. latifrons and B. dorsalis,5 whilst amides 1 and 3 were identified in the male secretions.
 | ||
| Fig. 1 Female B. tryoni volatile components. | ||
More interestingly, however, enantioselective GCMS analysis of the pentane extract of whole, crushed female abdomens revealed a suite of spiroacetals (Fig. 2), along with their approximate abundances. The major component (F) (∼83%) was identified as (2S,6R,8S)-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane (2) (Fig. 1) with the absolute configuration being determined by comparison of retention times and co-injection studies with authentic standards.6 The enantiomeric excess of (2S,6R,8S)-2 was found to be 98%, with its enantiomer being present at less than 1% (Fig. 2, Peak E), as previously reported for B. cucumis.7 The (E,Z)‡ isomers (2S,6S,8R)-6 and (2R,6R,8S)-7 were also identified (Fig. 2, Peaks L and M respectively), based on the order of enantiomer elution previously determined by McErlean.8 Peaks K and P were determined to be (2S,6R,8S)-4 (5%) and (2S,6R,8S)-5 (< 1%) respectively, with the absolute configurations of both being established by comparisons of retention times and co-injection of synthetic standards.6 With access to mass spectra of authentic compounds,9 peaks A–D and H were identified as isomers of either the C10 spiroacetal, 2,7-dimethyl-1,6-dioxaspiro[4.5]decane, or the C9 spiroacetal, 2-methyl-1,6-dioxaspiro[4.5]decane, and peaks G and J as the (E,E)- and (E,Z)-isomers‡ of 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]undecane. Peaks I (∼1%) and N (< 1%) were identified as nonanal and decanal.
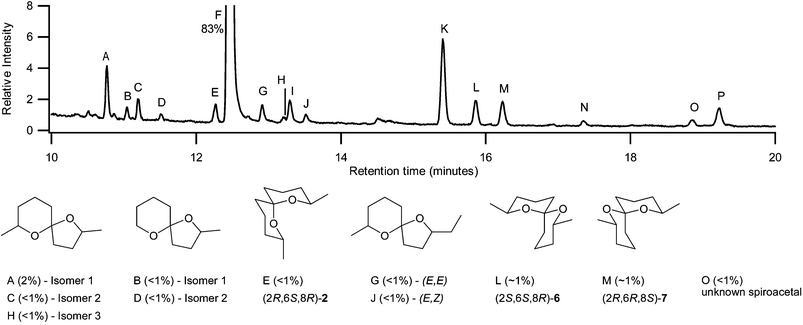 | ||
| Fig. 2 Enantioselective GCMS of pentane extract of female B. tryoni abdomens. Peak F set to 100 units. | ||
The spiroacetal generating peak O (< 1%), however, was more difficult to identify as its mass spectrum did not match that of any known spiroacetal.5 Close analysis indicated that the new spiroacetal had an apparent molecular ion of 212 (Fig. 3). The fragment ions at m/z 197, from loss of a methyl substituent, and m/z 168 from extrusion of ethanal, along with the ions at m/z 112, 115 and 125 are suggestive of a methyl substituted six-membered ring. The ions at m/z 140 and 143 are suggestive of a C8 pyran moiety, with the ion at m/z 183 indicative of ethyl loss. However the lack of a fragment ion at m/z 154 for the extrusion of propanal suggests the spiroacetal incorporates a tertiary ether centre. Indeed, if this carbon is substituted with both an ethyl and a methyl group, extrusion of 2-butanone would result in the observed fragment at m/z 140. Based on these considerations, the spiroacetal was tentatively proposed to be the previously unreported branched chain spiroacetal 2-ethyl-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane (8).
 | ||
| Fig. 3 GCMS and fragmentation analysis for spiroacetal O. | ||
Although this proposed spiroacetal is structurally different from the other spiroacetals found in B. tryoni, and no branched chain spiroacetal has been reported from Dipteran species, there is precedent for this spiroacetal type from Coleopteran insects. (6R,8S)-2,2,8-trimethyl-1,7-dioxaspiro[5.5]undecane (9) has been identified from Ontholestes murinus, and 2,4,8-trimethyl-1,7-dioxaspiro[5.5]undecane and 2,3,7-trimethyl-1,6-dioxaspiro[4.5]decane have been identified from Cantao parentum.10,11
In order to confirm the structure of 8, it was synthesised in racemic form as outlined in Scheme 1. Tertiary alcohol 10 (obtained from addition of pent-4-enylmagnesium bromide to 2-butanone) was protected as the TBS ether and ozonolysed to afford aldehyde 11. Addition of key alkyne 126 generated propargylic alcohol 13, which after PDC oxidation to ketone 14 and reduction of the triple bond was cyclised under acidic conditions. The resulting inseparable 1 : 1 mixture of two isomers of spiroacetal 8 was purified by preparative GC.
 | ||
| Scheme 1 Synthesis of 8. Reagents and conditions: i) KH, 18-crown-6, TBSCl, THF; ii) O3, CH2Cl2, −78 °C, PPh3, 70% over two steps; iii) 12, BuLi, THF, −78 °C, 76%; iv) PDC, CH2Cl2, 92%; v) H2(g), Pd/C, THF, 89%; vi) 75% AcOH, 60 °C, 16%. | ||
Both isolated isomers displayed mass spectra very similar to that of O from the natural extract and were expected to be the two possible isomers (Fig. 4, 15 and 16), with both oxygens axially directed to provide maximum anomeric stabilisation. These isomers (15 and 16) differ only in the orientation of the C2 substituents. Formation of isomers, such as 17 (Fig. 4), that are normally observed in disubstituted spiroacetals in which the methine carbons possess differing configurations, e.g. (E,Z)-6,‡ was unlikely. (Such isomers lack one anomeric stabilisation and normally form to relieve 1,3-diaxial strain resulting from an axial alkyl substituent). In the case of spiroacetal 8, reversal of the disubstituted ring would result in interchanging an axial ethyl group for an axial methyl group. Given the small difference in A values for these two substituents (1.74 kcal mol−1 for methyl compared with 1.79 kcal mol−1 for ethyl),12 it was considered unlikely that any stabilisation gained from ring-reversal would offset the decrease in anomeric stabilisation.
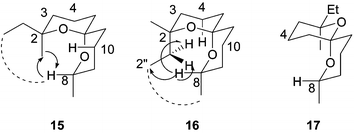 | ||
| Fig. 4 NOE interactions in spiroacetal isomers. | ||
Indeed, extensive NMR analyses confirmed that the isomers have different orientations of the methyl and ethyl groups but both possess maximum anomeric stabilisation. Analysis of the H-8 multiplets of both isomers revealed one large and one small coupling constant, as well as the coupling to the C(8) methyl (dqd, J 11.3, 6.3, 2.1 Hz), consistent with an axial proton and confirming that the C(8) methyl substituent was equatorial in both isomers. The downfield shifts for these H-8 protons (δ 3.93 and 4.02) also indicate that 1,3-diaxial interactions with oxygen rather than with carbon are operative as the former produces a greater downfield shift.10 This, in addition to the fact that the axial H-4 and H-10 protons of both isomers also display downfield shifts for the same reason, confirms that the two spiroacetal isomers adopt a configuration with the maximum anomeric stabilisation. As anticipated, the proton and carbon chemical shifts for the two isomers were very similar except for those for C3, C2 and the C2 methyl and ethyl substituents. 1-D and 2-D NOESY spectra established that one isomer shows a strong NOE between H-8 (δ 3.93) and a methyl singlet (δ 1.35), with another, much weaker NOE to a methyl triplet (δ 0.97), indicating an axial methyl and equatorial ethyl arrangement as in 15 (Fig. 4). The other isomer, however, shows strong NOEs between H-8 (δ 4.02) and a methyl triplet (δ 0.81), and two chemically inequivalent methylene protons (δ 2.28 and 1.69), indicating that this isomer possesses an axial ethyl and equatorial methyl arrangement (Fig. 4, 16). This is also confirmed by the absence of any NOE from H-8 (δ 4.02) to the methyl singlet (δ 1.18).
Fortuitously, flash chromatographic purification of the spiroacetals provided a fraction with unequal proportions of the two isomers. Proton NMR analysis confirmed a 30 : 70 ratio of isomers, in favour of 15. This allowed the structure of the isomers to be matched with their GC retention times and their mass spectra. Close inspection of the mass spectral fragmentations (Fig. 5a and b) shows that isomer 16 consistently has a more intense m/z 183 ion than m/z 112, whereas isomer 15 has a more intense m/z 112 than m/z 183. Comparisons of these spectra to that of O, which clearly has a more intense m/z 112 than m/z 183, indicated that the natural spiroacetal is isomer 15 with equatorial ethyl and axial methyl groups.
 | ||
| Fig. 5 (a) Mass spectrum of 16. (b) Mass spectrum of 15. | ||
Under our enantioselective GC conditions, spiroacetals with (S)-spirocentres elute prior to those with (R)-spirocentres. This is observed with the enantiomers of 2, 6/7, 4, 5 and also with the branched spiroacetal 9 and its enantiomer.10 On this basis and by comparison of the retention times of the fraction containing an unequal proportion of 15 and 16 with that of O, it was possible to determine tentatively that the novel spiroacetal identified in the extract from female B. tryoni is (2S,6R,8S)-2-ethyl-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane (15).
In conclusion, a diverse suite of spiroacetals from the volatile secretions of female B. tryoni has been identified, which ranges from nine to thirteen carbons and includes unusual even carbon numbered spiroacetals, as well as a novel branched chain spiroacetal. Spiroacetal biosynthesis in Bactrocera species is proposed to involve P450 catalysed hydroxylation of an alkyltetrahydropyranol, followed by cyclisation13 and it will be of much interest to determine whether B. tryoni produces such a diverse range of spiroacetals in the same way.
The authors are grateful for IPRS scholarship support for Y. Booth.
Notes and references
- E. Colquhoun, Review and Management Strategy for R&D on Control of Fruit Fly in the Field. Horticultural Research and Development Corporation Final Report on Project CT97024, Gordon, NSW, 1998 Search PubMed.
- R. T. Carde and A. K. Minks, Annu. Rev. Entomol., 1995, 40, 559–585 CrossRef CAS.
- T. E. Bellas and B. S. Fletcher, J. Chem. Ecol., 1979, 5, 795–803 CAS.
- M. T. Fletcher and W. Kitching, Chem. Rev. (Washington, DC, US), 1995, 95, 789–828 Search PubMed.
- W. Francke and W. Kitching, Curr. Org. Chem., 2001, 5, 233–251 CrossRef CAS.
- B. D. Schwartz, P. Y. Hayes, W. Kitching and J. J. De Voss, J. Org. Chem., 2005, 70, 3054–3065 CrossRef CAS.
- W. Kitching, J. A. Lewis, M. V. Perkins, R. Drew, C. J. Moore, V. Schurig, W. A. Konig and W. Francke, J. Org. Chem., 1989, 54, 3893–3902 CrossRef CAS.
- C. S. P. McErlean, M. T. Fletcher, B. J. Wood, J. J. De Voss and W. Kitching, Org. Lett., 2002, 4, 2775–2778 CrossRef CAS; C. S. P. McErlean, PhD Thesis, University of Queensland, 2002.
- J. A. Lewis, PhD Thesis, University of Queensland, 1987.
- Y. Q. Tu, A. Hubener, H. Zhang, C. J. Moore, M. T. Fletcher, P. Hayes, K. Dettner, W. Francke, C. S. P. McErlean and W. Kitching, Synthesis, 2000, 1956–1978 CrossRef CAS.
- P. Hayes, M. T. Fletcher, C. J. Moore and W. Kitching, J. Org. Chem., 2001, 66, 2530–2533 CrossRef CAS.
- H. Booth and J. R. Everett, J. Chem. Soc., Perkin Trans. 2, 1980, 255–259 RSC.
- J. E. Stok, C.-S. Lang, B. D. Schwartz, M. T. Fletcher, W. Kitching and J. J. De Voss, Org. Lett., 2001, 3, 397–400 CrossRef CAS; M. T. Fletcher, B. E. Mazomenos, J. H. Georgakopoulos, M. A. Konstantopoulou, B. J. Wood, J. J. De Voss and W. Kitching, Chem. Commun., 2002, 1302–1303 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: GC conditions, synthesis and characterisation of spiroacetals 15 and 16. See DOI: 10.1039/b611953k |
| ‡ Substituted spiroacetals are designated (E)- when the substituent group and oxygen atom of the alternate ring are on opposite sides of the reference plane (the substituted ring) and (Z) when on the same side. |
| This journal is © The Royal Society of Chemistry 2006 |

