Sol–gel encapsulation extends diatom viability and reveals their silica dissolution capability†
Clémentine
Gautier
ab,
Jacques
Livage
b,
Thibaud
Coradin
b and
Pascal J.
Lopez
*a
aDiatom Signaling and Morphogenesis, CNRS FRE-2910, Ecole Normale Supérieure, 75005 Paris, France. E-mail: pjlopez@biologie.ens.fr; Fax: +33 1 4432 3540; Tel: +33 1 4432 3535
bChimie de la Matière Condensée de Paris, CNRS UMR-7574, Université Pierre et Marie Curie, 75005 Paris, France
First published on 25th September 2006
Abstract
Several strains of diatom exhibit a long-term viability in silica gels and demonstrate the ability to dissolve the silica in their surroundings.
It is now well-accepted that sol–gel matrices can be used to entrap living organisms, such as yeasts, bacteria, animal or plant cells to design cell-based biosensors and bioreactors.1–3 However, an important future issue in sol–gel encapsulation processes could be to develop new hybrid materials whose properties could be controlled or modified by the entrapped organisms.
We hypothesized that because diatoms require silicon for growth they could be specifically adapted to sustain encapsulation within silica matrices, and might be good candidates to interact with the minerals found in these environments. These unicellular algae are interesting because they have important ecological roles, being responsible for about 20% of the annual net primary production on Earth (which corresponds to the production of organic compounds from atmospheric or aquatic carbon dioxide), and being a main component of the biogenic silicon cycle.4,5 Diatoms are also fascinating for the high degree of complexity displayed by their silica shells, for the potential applications of silica-precipitating proteins extracted from them, or for biomimetic approaches.6–10
On the basis of previous results on enzyme and bacteria encapsulation,11,12 a silicate-based approach was developed for the encapsulation of diatoms. Sodium silicate solutions were diluted in demineralized water in the 0.25 M–1 M range, and HCl (4 M) was added until neutralization. A suspension of diatoms in their culture medium was then added. Once the gel was formed, artificial sea water was deposited on the gel surface. For concentrations of 0.75 M or above, the gelation time was too fast to allow homogeneous dispersion of the cells in the condensing media. Moreover, rapid cell death was observed as a probable consequence of the gel formation process. For a 0.25 M silicate concentration, gelation times were more than 10 min but the resulting network was found to be too soft to hinder cell motility, resulting in diatoms leaching out of the gel. Finally, the silica gel concentration was adjusted to 0.5 M, which is a compromise between fast gel time formation (tg = 240 ± 40 s, measured by a Couette shear cell) and appropriate gel stiffness.
As an indicator of cell viability, the photosynthetic activity of the entrapped diatoms was followed using the PAM (pulse amplitude modulated fluorometry) technique.13 This technique measures the optimum photosynthetic quantum yield Fv/Fm, an indicator of the number of photosystem II reaction centers.‡ This non-invasive technique is widely used to study photosynthetic organisms, including diatoms, and can be performed in situ because of the transparency of the silica gel.
We first studied the diatom Cylindrotheca fusiformis, a representative of the pennate class characterized by elongate cells with bi-lateral symmetry (Fig. 1).14 This diatom is also well-known for biochemical studies of organic compounds involved in silica biomineralization.15 At the beginning of the experiments, the maximum quantum efficiency of PSII photochemistry (Fv/Fm) was 0.70 ± 0.01 (Fig. 1), which corresponds to the maximum Fv/Fm for this strain, indicating that the cells were in optimum physiological conditions.
 | ||
| Fig. 1 Comparison of the optimum quantum yield (Fv/Fm) of encapsulated diatoms. Cylindrotheca fusiformis in silica matrix (C.f., black rhombus) or in solution (black crosses). The other diatoms successfully encapsulated are Phaeodactylum tricornutum (P.t., light grey rings) and Thalassiosira weissflogii (T.w., grey squares). Each time point corresponds to the average of 2 to 5 independent experiments performed in duplicate. The s.d. is 4–16% of the mean. The differential interference contrast images (top right) illustrate the morphology of the strains. | ||
Subsequently, time-course measurements revealed a steady decrease of Fv/Fm. However, we calculated that compared to in-gel conditions, the slope of Fv/Fm was 2.8 steeper when cells were in liquid culture (Fig. 1). A possible explanation for the decrease in Fv/Fm at such high cell-density (2 × 107 cells ml−1 in-gel compared with ca. 106 cells ml−1 in batch cultures) is that the cells might exhaust the available nutrients (e.g. supply of inorganic carbon, nitrogen, etc.) or that the population begins to age. Interestingly, for in-gel diatoms the decrease in Fv/Fm was slower when both the growth temperature and the light intensity were reduced (not shown). Furthermore, C. fusiformis cells could be recovered by spreading the gel on appropriate agar plates (or in liquid medium) even after two months, when Fv/Fm had reached values as low as 0.001, revealing that at least a few cells were persistent for long periods of encapsulation times even though the detection of their PSII capabilities was below the PAM limits. We therefore propose that encapsulation leads to long-term protection of chloroplast activity and that helps the cells to cope with stresses resulting from culture conditions.
Encapsulation in silica gels was extended to other diatom species characterized by different cell morphologies and ecological niches. As another example of a pennate diatom, we chose the model species Phaeodactylum tricornutum, for which the genome has been completely sequenced and molecular tools exist.16 The culture used contained about 90% of the oval morphotype (capable of synthesizing a silica frustule) and ∼10% of fusiform cells. For P. tricornutum the quantum yield (Fv/Fm) declined over a period of more than 100 days (Fig. 1), illustrating that, compared to C. fusiformis, this strain has longer in-gel survival capacities. As a prototype centric species (cells with radial symmetry) we first chose the bloom forming diatom Thalassiosira weissflogii (ca. 10–25 µm). Starting from a Fv/Fm value of 0.70 ± 0.04 it took about 40 days before T. weissflogii PSII activity was no longer detectable (Fig. 1). A different result was obtained for other centric species. For Thalassiosira pseudonana (a small diatom for which the genome is known), Skeletonema costatum (a centric chain-forming diatom), and Ditylum brightwellii cell lysis occurred at the time of gel formation. Finally, the very large (>100 µm) benthic diatom, Coscinodiscus sp., showed variable behavior in different experiments with cell death sometimes occurring within the first few days or after a few weeks. Such behavior might imply that the physiological state of Coscinodiscus is particularly crucial for long-term cell survival. Altogether our experiments suggest that it is not the properties of the silica-frustule per se (significant mechanical strengths were shown to be required to break diatom cells17) but rather an intrinsic physiological aptitude that allows diatoms to survive encapsulation; i.e. to withstand external pressure at gel-time formation.
The encapsulation of diatoms also offers the opportunity to test whether these microorganisms can interact with the surrounding matrix. In-gel algae were therefore analyzed using ultrathin-sections followed by transmission electron microscopy (TEM).§ Because we wanted to analyze cells with high physiological activity, the observations were preferentially performed within the first week post-encapsulation. For C. fusiformis, during this period from 0–8 days the ratio Fv/Fm is maintained above 80% of the initial value. At the shortest time point tested, 10 min post-encapsulation, C. fusiformis cells are found surrounded by the silica gel, which is seen as an assemblage of silica colloidal particles (Fig. 2). Remarkably, at longer times, the gel in the vicinity of the cells was found to disappear and a non-mineralized area was visible around the cells (Fig. 2). Importantly, such disappearance of the gel was not observed when the cells were killed prior to encapsulation,† indicating that diatom physiological and/or protein activities are necessary to dissolve the silica gel.
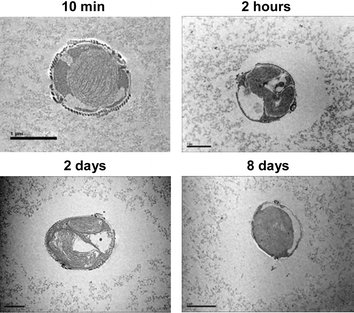 | ||
| Fig. 2 Dissolution of silica-colloidal particles by diatoms. TEM images of C. fusiformis (transversal view) entrapped in 0.5 M silica gel. The scale bar corresponds to 1 µm for 2 hours and 2 days, 5 µm for 10 min and 2 µm for 8 days. | ||
To quantify the dissolution process we developed image analyses, and determined the surface areas of the cell and of the dissolved-colloidal particles (Fig. 3, Insert). A simple dissolution index Idissolution which accounts for the position of the cell in the section, was then calculated according to:
| Idissolution = (Stotal − Scell)/Scell | (1) |
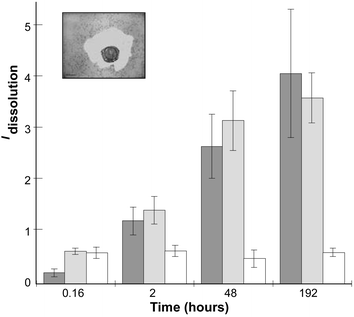 | ||
| Fig. 3 Measurement of the silica-gel dissolution index. The insert correspond to an image of C. fusiformis after 8 days of entrapment, and illustrates the measured surfaces of the cavity and of the cells. Idissolution is reported as a function of time. Light grey, C. fusiformis (n = 4); dark grey, P. tricornutum (n = 3); and white, Nannochloropsis salina (n = 2). In each experiment from 4 to 25 surface measurements were performed. Error bars indicate s.d. | ||
This approach was reproduced with P. tricornutum. For this diatom species, Idissolution also increased during the 8 days of recording (Fig. 3). However, such dissolution was not observed when similar experiments were performed with another alga, Nannochloropsis salina, from the same lineage of diatoms (heterokonts) but which is not silicified (Fig. 3). Our results strongly suggest that diatoms can specifically induce the dissolution of silica-colloidal particles in their vicinity.
An explanation for this dissolution process is that silicic acid taken-up by diatoms to form their silica shell could unbalance equilibriums favoring chemical dissolution. Soluble silicon concentrations (CSi) released by the gel, corresponding to monomeric and dimeric silicic acid, were measured using the blue silicomolybdic assay.¶18 From an initial CSi = 2.5 ± 0.1 mM, a slight increase was observed over time and concentrations of 4.5 ± 0.1 mM were reached after 8 days. Because similar concentrations were obtained in the absence of cells (CSi = 2.4 ± 0.1 mM and 4.1 ± 0.1 mM for 10 min after tg or 8 days later, respectively) we believed that such variation is probably due to partial degradation of the gel surface in contact with the supernatant medium. Nevertheless, such a soluble silicon pool far exceeds the needs of diatoms, arguing against a model of dissolution that is strictly coupled to diatom up-take. Another hypothesis that we favor is that silica particle dissolution could occur via secretion of specific organic compounds. To investigate the secretion of extracellular polymeric substances (EPS) inside the gels we stained thin-sections with ruthenium red, a cationic dye that specifically stains polysaccharides. In these conditions a network of secreted fibers spreading over the silica network could be visualized (Fig. 4); confirming that cellular biosynthesis and secretion occurs inside the gel.
 | ||
| Fig. 4 In-gel secretion of polysaccharides by C. fusiformis. | ||
However, attempts to extract the organic material—proteins and carbohydrates—from the gel have so far been unsuccessful. More progress for the elucidation of the biochemical mechanisms involved in silicate dissolution by living diatoms is therefore needed. Nonetheless, it is worth mentioning that a “silicase” activity has recently been reported for another well-known species that performs silica biomineralization, the sponge Suberites domuncula.19
Our experiments are the first demonstration that diatoms have an extended photosynthetic activity when they are entrapped at high cell densities in silica gels. Moreover, they reveal that diatoms have the capability to dissolve silica present in their surrounding. To our knowledge, this possibility has never been addressed before and may correspond to a new biochemical process. Further demonstration of the silicate dissolution activity by diatoms might also have major implications for our understanding of the Si biogeochemical cycle.
Until now, silica gels have been used as physically and chemically inert hosts to stabilize living organisms. In the case of diatoms, it appears that encapsulated cells can also strongly modify the properties of the gel. We believe that this ability of diatoms to remodel silica should help to develop new scaffold materials from which the desired porosity and mechanical properties can be tuned by the entrapped organisms, opening perspectives for the development of new biotechnological and biomedical devices. In future, genetic engineering, already achieved for several diatoms,16 could also be exploited in conjunction with encapsulation to produce specific metabolites of interest.
Notes and references
- D. Avnir, T. Coradin, O. Lev and J. Livage, J. Mater. Chem., 2006, 16, 1013 RSC
.
- H. Böttcher, U. Soltmann, M. Mertig and W. Pompe, J. Mater. Chem., 2004, 14, 2176–2188 RSC
.
- G. Carturan, R. Dal Toso, S. Boninsegna and R. Dal Monte, J. Mater. Chem., 2004, 14, 2087–2098 RSC
.
- C. B. Field, M. J. Behrenfeld, J. T. Randerson and P. Falkowski, Science, 1998, 281, 237–240 CrossRef CAS
.
- P. Tréguer, D. M. Nelson, A. J. Van Bennekom, D. J. DeMaster, A. Leynaert and B. Quéguiner, Science, 1995, 268, 375–379 CAS
.
- K. F. Brandstadt, Curr. Opin. Biotechnol., 2005, 16, 393–397 CrossRef CAS
.
- P. J. Lopez, C. Gautier, J. Livage and T. Coradin, Curr. Nanosci., 2005, 1, 73–83 Search PubMed
.
- D. E. Morse, Trends Biochem. Sci., 1999, 17, 230–232 CAS
.
- S. V. Patwardhan, S. J. Clarson and C. C. Perry, Chem. Commun., 2005, 113 RSC
.
- M. Sumper, Angew. Chem., Int. Ed., 2004, 43, 2251–2254 CrossRef CAS
.
- R. B. Bhatia, C. J. Brinker, A. K. Gupta and A. K. Singh, Chem. Mater., 2000, 12, 2434–2441 CrossRef CAS
.
- N. Nassif, O. Bouvet, M. N. Rager, C. Roux, T. Coradin and J. Livage, Nat. Mater., 2002, 1, 42–44 CrossRef CAS
.
- G. Krause and E. Weis, Annu. Rev. Plant Phys., 1991, 42, 313–349 Search PubMed
.
-
F. E. Round, R. M. Crawford and D. G. Mann, The diatoms, Cambridge University Press, Cambridge, 1990 Search PubMed
.
- M. Sumper and N. Kröger, J. Mater. Chem., 2004, 14, 2059–2065 RSC
.
- P. J. Lopez, J. Descles, A. E. Allen and C. Bowler, Curr. Opin. Biotechnol., 2005, 16, 180–186 CrossRef CAS
.
- C. E. Hamm, R. Merkel, O. Springer, P. Jurkojc, C. Maier, K. Prechtel and V. Smetacek, Nature, 2003, 421, 841–843 CrossRef CAS
.
-
R. K. Iler, The chemistry of silica: solubility, polymerisation, colloid and surface properties, and biochemistry, Wiley-Interscience, New York, 1979 Search PubMed
.
- H. C. Schroder, A. Krasko, G. Le Pennec, T. Adell, M. Wiens, H. Hassanein, I. M. Muller and W. E. Muller, Prog. Mol. Subcell. Biol., 2003, 33, 249–268 Search PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: TEM images of encapsulated dead cells. See DOI: 10.1039/b609121k |
| ‡ The measurements were performed on a PAM 101 apparatus from Walz. The photosynthesis quantum yield is defined as: Fv/Fm = (Fm − F0)/Fm where Fv is the variable fluorescence, F0 is the PSII fluorescence under a low-intensity light modulated at 1.6 kHz from a light-emitting diode working at 655 nm and Fm the maximum PSII fluorescence after a 1 s flash of saturating white light. |
| § In-gel diatoms were first fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) at 4 °C. Postfixation was then performed in 1% aqueous OsO4, and finally, after dehydration the samples were embedded in araldite resin. Ultrathin sections were obtained with an Ultracut microtome, and inspections were performed with a Philips CM12 electron microscope. The area (µm2) of the cell, Scell, or of the empty gel + cell, Stotal, was determined with image-analysis software (MetaMorph Imaging System). |
| ¶ Titration was performed on 400 µl of the gel supernatant diluted in a suitable volume of deionized water; the CSi values fell into the method linear calibration range, i.e. 0.5–0.05 mM. |
| This journal is © The Royal Society of Chemistry 2006 |
