Ionic liquids enable electrospray ionisation mass spectrometry in hexane†
Matthew A.
Henderson
and
J. Scott
McIndoe
*
Department of Chemistry, University of Victoria, P.O. Box 3065, Victoria, BC V8W 3V6, Canada. E-mail: mcindoe@uvic.ca; Fax: +1 (250) 721-7147; Tel: +1 (250) 721-7181
First published on 16th June 2006
Abstract
Addition of a lipophilic ionic liquid to non-polar, hydrocarbon solvents (such as hexane and toluene) permits electrospray ionisation mass spectrometric analysis of dissolved analytes.
Electrospray ionisation mass spectrometry (ESI-MS) has made possible the swift characterisation of a huge range of thermally labile, polar molecules, most notably biomolecules but also pharmaceuticals,1 coordination complexes,2 supramolecular assemblies3 and organometallic catalysts.4 The ESI-MS process transfers ions, pre-formed in solution, into the gas phase. Neutral compounds, unless they interact with charged species such as H+ or Na+, are essentially invisible to ESI-MS. A limitation of the ESI technique has been choice of solvent; it is generally only reasonably volatile, polar solvents that provide a stable spray and satisfactory spectra. Water, methanol and acetonitrile are most commonly employed, frequently with organic acids added to enhance the formation of protonated species. Less polar solvents such as CH2Cl2 provide good spectra under the right conditions,5 but we are unaware of any reports of ESI mass spectra having been successfully recorded in non-polar solvents such as hexane. We report herein a convenient means of enabling ESI-MS to be performed in a variety of non-polar hydrocarbon solvents, by simply adding a small amount of a lipophilic ionic liquid.
The main problem that prevents non-polar solvents being used in ESI-MS may simply be lack of conductivity. ESI-MS is fundamentally an electrochemical process, with the same number of ions being discharged at the capillary as are generated in the source.6 Non-polar solvents may shut down this process due to high resistance, a problem which is dealt with in electrochemistry by the addition of a supporting electrolyte.7 Supporting electrolytes in conjunction with electrochemical processes in electrospray have garnered attention before, but the emphasis has been on reducing the negative aspects of their presence, viz. suppression of a desired analyte by a buffer.8 Signal suppression by an abundance of electrolyte does not, however, appear to be a significant problem in some cases, e.g. the ESI-MS characterisation of organometallic complexes.9
We reasoned that a supporting electrolyte may well improve the solvent characteristics with respect to the electrospray process. A range of non-polar solvents become amenable to electrospray upon addition of a suitable salt; we used the commercially-available ionic liquid [PR13R2]+[N(SO2CF3)2]− (1) (R1 = hexyl, R2 = tetradecyl). An ionic liquid (IL) was chosen for several reasons: neat ILs themselves may be electrosprayed with the intention of characterising dissolved analytes10 (though it is likely that at least some molecular solvent is required to facilitate the process);11 a recent paper has shown that ILs can be electrosprayed successfully from a benzene solution;12 and there is a huge selection of commercially available ILs with a wide variety of properties, including miscibility with hexane. ILs have previously found utility in mass spectrometry as matrices for matrix-assisted laser desorption ionisation (MALDI).13
ESI-MS‡ of pentane, hexane and cyclohexane solutions of 1 do not provide a stable spray (manifested by spectra that consist entirely of noise) until a concentration of ∼10−5 M is reached, at which point excellent spectra of the species [CnAn−1]+ (where C = cation and A = anion, positive ion mode, see Fig. 1) and [Cn−1An]− (negative ion mode) (n = 1 or 2) are obtained.
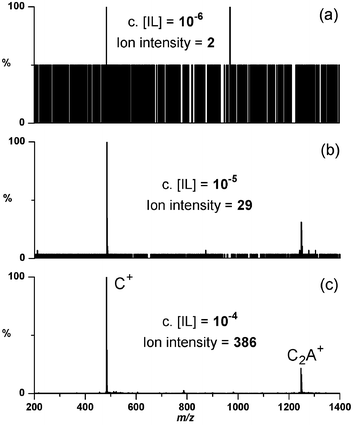 | ||
| Fig. 1 Positive-ion ESI mass spectra of 1 at differing concentrations in cyclohexane. Each spectrum was collected over 1 minute (60 summed spectra). A stable spray is obtained at a threshold of ∼10−5 mol L−1. | ||
Benzene and toluene require slightly lower concentrations of the ionic liquid (see the Supporting Information for full details), and for more polar solvents (e.g. dichloromethane, diethyl ether, fluorobenzene), we were untroubled in collecting good spectra of the IL down to the detection limits of the instrument; there appeared to be no minimum concentration of IL required for a stable spray.
A further advantage of 1 is its high molecular weight, resulting in aggregates (in the positive ion mode, [CnAn−1]+, in the negative ion mode [Cn−1An]−) spaced 763 m/z apart, leaving wide, clear windows for analytes in which the salt-derived ions do not interfere. Only two ions from the ionic liquid appear in most spectra: the bare ion and the first aggregate. The high molecular weight, lipophilic salt [PR13R2]+[BArF4]− (ArF = 3,5-bis{trifluoromethyl}phenyl) (2)§ contains a very non-coordinating anion and may prove to be a good choice when examining highly reactive systems. It is even better than 1 in terms of limiting interference in the mass spectrum, as the gap between aggregates is 1346 m/z, and in practice, the higher aggregates are barely detectable (Fig. 2). This observation is in stark contrast to simple salts such as sodium iodide, in which the aggregates form with such ease that the series [NanIn−1]+ and [Nan−1In]− are routinely used for instrument calibration. ESI-MS studies of imidazolium-based ionic liquids reveal similarly extensive supramolecular assemblies of the IL.14
 | ||
| Fig. 2 Negative-ion ESI-MS of 2 in toluene. The region between 2000 and 2400 m/z has been enhanced in intensity by a factor of 500. | ||
2 is soluble in toluene but phase-separates with hexane; however, it is sufficiently soluble in hexane to enable the electrospray process to work well. Another readily-prepared salt suitable for enabling ESI-MS in non-polar solvents is [(C2H4O)6Na]+[BArF4]− (3), made by adding a stoichiometric amount of Na[BArF4] to a solution of 18-crown-6 (see Supporting Information for spectrum).
Of course, enabling a stable spray in a non-polar solvent means that solvent may be used in routine fashion for the characterization of charged solution species. Adulterating solvents for enhanced spray and ionization characteristics is nothing new; acids are routinely added, solvent mixtures are frequently employed, buffered solutions are sprayed, with the intention of either improving sensitivity or convenience. Here, the salt acts as an enabling additive in a similar fashion, and provided the analyte of interest does not have an ion coincident in m/z with the salt, analysis can proceed without difficulty. An example of this can be seen in Fig. 3 for [Rh(COD)(PPh3)2][BF4]. The spectrum, whilst dominated by peaks associated with 1, provides high quality data on the analyte of interest. If so desired, spectra uncomplicated by the IL may be obtained by background subtraction of a blank.
![Positive-ion ESI-MS of [Rh(COD)(PPh3)2]+ in cyclohexane/1. Inset: expansion of isotope pattern match for the analyte.](/image/article/2006/CC/b606938j/b606938j-f3.gif) | ||
| Fig. 3 Positive-ion ESI-MS of [Rh(COD)(PPh3)2]+ in cyclohexane/1. Inset: expansion of isotope pattern match for the analyte. | ||
The IL acts to assist collection of ESI-MS in an additional way: ionic complexes of low solubility dissolve much more readily in the IL-adulterated solvent, presumably because they undergo counterion exchange. An especially obvious example of this behavior may be observed for [HNEt3][HFe3(CO)11],15 which is insoluble in hexane but dissolves readily in hexane containing 1 (Fig. 4).
![Negative-ion ESI-MS of [NEt3H][HFe3(CO)11] in hexane/1 (n = 8–11). Inset: while the compound is hexane-insoluble (left), it dissolves readily when 10−4 mol L−1 of 1 is added (right).](/image/article/2006/CC/b606938j/b606938j-f4.gif) | ||
| Fig. 4 Negative-ion ESI-MS of [NEt3H][HFe3(CO)11] in hexane/1 (n = 8–11). Inset: while the compound is hexane-insoluble (left), it dissolves readily when 10−4 mol L−1 of 1 is added (right). | ||
JSM thanks the Canada Foundation for Innovation (CFI), the British Columbia Knowledge Development Fund (BCKDF) and the University of Victoria for a New Opportunities Fund Award, and the Natural Sciences and Engineering Research Council (NSERC) of Canada for a Discovery Grant. We thank Dr Andrew Weller (Bath) for the Na[BArF4] and Sarah Jackson (Victoria) for a sample of [Rh(COD)(PPh3)2][BF4].
Notes and references
- Electrospray Ionization Mass Spectrometry, ed. R. B. Cole, Wiley, New York, 1997 Search PubMed.
- Mass Spectrometry of Inorganic and Organometallic Compounds, W. Henderson and J. S. McIndoe, Wiley, Chichester, 2005 Search PubMed.
- A. Di Tullio, S. Reale and F. De Angelis, J. Mass Spectrom., 2005, 40, 845 CrossRef CAS.
- R. A. J. O'Hair, Chem. Commun., 2006, 1469 RSC; P. Chen, Angew. Chem., Int. Ed., 2003, 42, 2832 CrossRef CAS.
- S. K. Brayshaw, M. Ingleson, J. C. Green, P. R. Raithby, G. Kociok-Köhn, J. S. McIndoe and A. S. Weller, Angew. Chem., Int. Ed., 2005, 44, 6875 CrossRef CAS.
- J. F. de la Mora, G. J. Van Berkel, C. G. Enke, R. B. Cole, M. Martinez-Sanchez and J. B. Fenn, J. Mass Spectrom., 2000, 35, 939 CrossRef CAS; P. Kebarle, J. Mass Spectrom., 2000, 35, 804 CrossRef CAS.
- Electrochemical Systems, J. Newman and K. E. Thomas-Alyea, Wiley, Hoboken, 2004 Search PubMed.
- I. Tang and P. Kebarle, Anal. Chem., 1993, 65, 3654 CrossRef CAS; A. M. Bond, R. Colton, A. D'Agostino, A. J. Downard and J. C. Traeger, Anal. Chem., 1995, 67, 1691 CrossRef CAS; F. Zhou and G. J. Van Berkel, Anal. Chem., 1995, 67, 3643 CrossRef CAS.
- P. J. Dyson, J. S. McIndoe and D. Zhao, Chem. Commun., 2003, 508 RSC.
- G. P. Jackson and D. C. Duckworth, Chem. Commun., 2004, 522 RSC.
- P. J. Dyson, I. Khalaila, S. Luettgen, J. S. McIndoe and D. Zhao, Chem. Commun., 2004, 2204 RSC.
- J. Łachwa, J. Szydłowski, A. Makowska, K. R. Seddon, J. M. S. S. Esperança, H. J. R. Guedes and L. P. N. Rebelo, Green Chem., 2006, 8, 262 RSC.
- D. W. Armstrong, L. K. Zhang, L. He and M. L. Gross, Anal. Chem., 2001, 73, 3679 CrossRef CAS.
- F. C. Gozzo, L. S. Santos, R. Augusti, C. S. Consorti, J. Dupont and M. N. Eberlin, Chem.–Eur. J., 2004, 10, 6187 CrossRef CAS.
- W. MacFarlane, G. Wilkinson and W. Hübel, Inorg. Synth., 1966, 8, 182.
Footnotes |
| † Electronic supplementary information (ESI) available: General experimental details. ESI-MS of 1 at various concentrations in pentane, cyclohexane, hexane, toluene and benzene. Synthesis and characterization of 2. ESI-MS of 3 in toluene. See DOI: 10.1039/b606938j |
| ‡ ESI-MS data were collected on a Waters® Micromass® Q-Tof micro™ mass spectrometer with Z-spray electrospray source. Samples were infused from a 250 µL gas-tight syringe at 10–40 µL min−1, via a syringe pump. Instrument settings were unexceptional: capillary voltage 2900 V, cone voltage 20 V, source temperature 100 °C, desolvation gas temperature 200 °C. Nitrogen was used as the desolvation gas. |
| § Synthesis of 2. Trihexyltetradecylphosphonium chloride (0.143 g, 0.28 mmol) was dissolved in toluene (4 mL) and added to a solution of sodium tetra-(3,5-bis(trifluoromethyl)phenyl)borate (0.243 g, 0.28 mmol) in toluene (6 mL) with two drops of acetonitrile. The mixture was stirred for one hour, filtered through a Celite plug and washed with toluene. The solvent was removed and the residue dried under vacuum at 50 °C for 3 hours to provide 2 (42%, not optimized). 1H NMR (CDCl3): δ (ppm) 7.68 (s, 8H, ortho-H); 7.51 (s, 4H, para-H); 1.92, 1.56, 1.42, 1.23, 0.87, 0.85 (all multiplets, 68H, CH2 and CH3). ESI-MS: (+ve) 483.5 m/z [PC32H68]+; ESI-MS (−ve) 863.1 [BC32H12F24]−. |
| This journal is © The Royal Society of Chemistry 2006 |
