Selective recognition of pyrophosphate in water using a backbone modified cyclic peptide receptor†
Matthew J.
McDonough
,
Aaron J.
Reynolds
,
Wee Yu Gladys
Lee
and
Katrina A.
Jolliffe
*
School of Chemistry, The University of Sydney, 2006, NSW, Australia. E-mail: jolliffe@chem.usyd.edu.au; Fax: +61 2 9351 3329; Tel: +61 2 9351 2297
First published on 22nd June 2006
Abstract
A cyclic peptide based receptor, bearing two dipicolylamino arms complexed to zinc(II) ions, binds pyrophosphate ions with high affinity and selectivity in aqueous solution as determined using an indicator displacement assay.
The selective recognition and sensing of biologically important anions is of intense current interest to both chemists and biologists. Anions such as pyrophosphate (P2O74−, PPi) play important roles in bioenergetic and metabolic processes.1 Therefore, the selective detection of these anions under physiological conditions has numerous applications in biomedicine. However, there are relatively few reports of pyrophosphate-selective receptors or sensors that operate in aqueous solution.2,3
Recently, analogues of the naturally occurring lissoclinum family of cyclic peptides4 have been proposed to be useful structures for the construction of molecular receptors.5 The incorporation of oxazole units into the peptide backbone increases the rigidity of the cyclic peptide scaffold and if the side chains are all of the same configuration, they are presented on the same face of the molecule in a convergent manner for binding. We now report the first example of an anion receptor, based on a lissoclinum-type cyclic peptide scaffold, and bearing two pendant dipicolylamino (Dpa) arms complexed to zinc(II) (1·Zn2). In addition we demonstrate that this receptor can be utilised in a fluorescent ‘chemosensing ensemble’ with coumarin derivative 2, exhibiting high sensitivity and selectivity for PPi anions under physiological conditions (pH 7.2, 5 mM HEPES, 145 mM NaCl). This indicator–displacement sensing strategy relies upon displacement of a non-covalently attached fluorescent indicator from an indicator–receptor complex by the analyte ion,6 thus avoiding the need for covalently linking the receptor and fluorophore.
Artificial receptors bearing two Zn(II)–Dpa groups have recently been found to show high affinity towards phosphate derivatives in aqueous solution.3,7,8 To improve selectivity for PPi over other phosphate oxoanions (e.g., HPO42−, ATP and ADP), the cyclic octapeptide 1 was chosen as a scaffold to position two Zn(II)–Dpa groups at an appropriate distance to complement the size and geometry of the pyrophosphate anion, with each zinc(II) centre expected to bind to two oxygen atoms of the PPi ion. Scaffold 3, bearing Cbz-protected ornithine side chains was readily synthesised upon macrocyclisation of the corresponding linear precursor.9 Cleavage of the Cbz groups, followed by reductive amination with 2-pyridinecarboxaldehyde gave 1 in good overall yield (Scheme 1).10
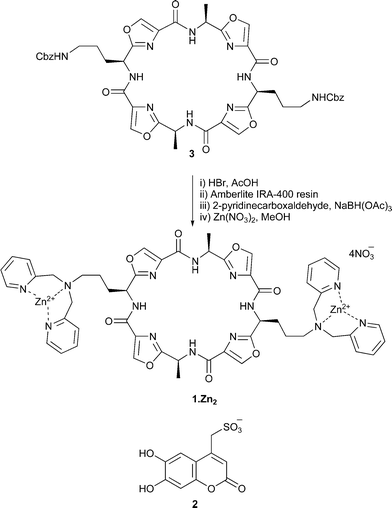 | ||
| Scheme 1 | ||
While it has previously been reported that lissoclinum-type cyclic peptides are capable of binding Zn(II) ions with complexation constants of 103–104 M−1,11 Dpa ligands are known to bind Zn(II) ions two to three orders of magnitude more strongly (106 M−1).8 Therefore, it was anticipated that addition of two equivalents of Zn(NO3)2 would result in the formation of the bis-Zn(II) complex 1·Zn2 in which the zinc ions are bound by the Dpa ligands. This was confirmed by titration of Zn(NO3)2 into a methanolic solution of 1 (see ESI†). The changes in chemical shift for the protons associated with the Dpa ligands clearly show that these ligands are involved in metal binding. In particular, the signal attributable to the benzylic protons is shifted downfield by 0.5 ppm and exhibits changes in the coupling pattern indicative of restricted rotation. Minor changes in chemical shift (<0.05 ppm) are also observed for the oxazole protons directly attached to the cyclic peptide scaffold.‡ However, no further changes are observed upon addition of more than two equivalents of Zn(NO3)2 indicating that the bis-zinc complex 1·Zn2 is formed. For use in our anion binding experiments we prepared 1·Zn2 by addition of two equivalents of Zn(NO3)2 to a solution of 1 in methanol. After removal of the solvent, the complex was redissolved in the appropriate solvent.
After screening a number of fluorescent and colorimetric indicators, we chose to examine the anion binding capabilites of 1·Zn2 in a fluorescence displacement assay using the coumarin derivative 2. This indicator has recently been used in displacement assays to detect low concentrations of pyrophosphate under physiological conditions3 and to detect phosphatidylserine in a bilayer membrane surface.12 We found that 1·Zn2 was capable of quenching the fluorescence emission of 2 (10 µM) in a concentration dependent fashion at 25 °C in an aqueous solution (buffered at pH 7.2 using 5 mM HEPES and 145 mM NaCl).§ A Job plot indicated 1 ∶ 1 binding stoichiometry and we determined an association constant log Kin = 5.1 ± 0.1 by fluorescence titration and subsequent non-linear curve fitting to a standard 1 ∶ 1 binding model (Fig. 1).
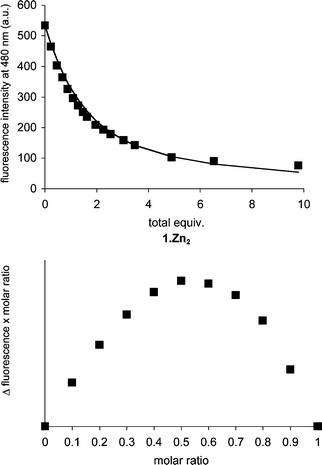 | ||
| Fig. 1 Top: Fluorescence intensity of 2 at 480 nm in the presence of increasing amounts of 1·Zn2. Excitation wavelength was 347 nm. Bottom: Jobs plot for the receptor indicator binding of 1·Zn2 and 2, clearly indicating a 1 ∶ 1 stoichiometry. | ||
In our assay for anions, we prepared a 1 ∶ 1 receptor ∶ indicator chemosensing ensemble by mixing equimolar amounts of 1·Zn2 and 2 (10 µM each) in aqueous solution. We then titrated this solution with aliquots of the sodium salts of nitrate, acetate, hydrogensulfate, iodide, phosphate, hydrogenphosphate, phenylphosphate, phosphotyrosine, phosphothreonine, phosphoserine, AMP, ADP, ATP, PPi and citrate. Only PPi, and to a lesser extent ATP, ADP and citrate were able to displace the indicator from the indicator ∶ receptor complex as indicated by increases in fluorescence intensity upon addition of these anions (see ESI†). Addition of greater than 10 equivalents of other anions (e.g., hydrogen phosphate) resulted in no change in the fluorescence of the chemosensing ensemble. Remarkably, almost complete fluorescence intensity was restored upon addition of a single equivalent of PPi. Fluorescence emission intensities (λex = 347 nm, λem = 480 nm) from these titrations were analysed using a curve-fitting procedure based on the equilibria previously described for competition assays,13 with our pre-determined value for Kin to determine the binding constants (Table 1). 1·Zn2 was found to bind PPi with a log K = 8.0 ± 0.1; at least two orders of magnitude more tightly than ATP or ADP are bound, indicating that 1·Zn2 has a high selectivity for PPi over other anions. Binding of monophosphate oxoanions was not detected using this chemosensing ensemble. The magnitude of the binding constant for PPi reflects the complementarity between the size and geometry of the cyclic peptide scaffold and that of the PPi anion. In particular, the increased distance between the two zinc(II) ions on the cyclic peptide scaffold, compared with that found in similar systems based on benzene scaffolds,3,7 results in selectivity for larger anions. The titration experiments indicate that a 10 µM solution of 1·Zn2 can detect PPi anions at nanomolar concentrations.
To determine why this system binds PPi with such high affinity and selectivity we examined the 1·Zn2·PPi complex by MS and NMR. The electrospray ionisation mass spectrum of the PPi complex exhibits two signals at m/z 1309 (minor) and 1331 (major), which correspond to [1·Zn2 + PPi + H]+ and [1·Zn2 + PPi + Na]+, respectively. The predominance of the second peak suggests that 1·Zn2 binds PPi in its fully deprotonated form. Addition of one equivalent of 1·Zn2 to a solution of tris(tetrabutylammonium) hydrogen pyrophosphate in CD3OD–D2O (1 ∶ 1 v/v) resulted in a shifting of the pyrophosphate 31P signals from −6.5 to −5.4 ppm, indicating that the biszinc complex interacts directly with the anions. The α-protons on the pyridine rings of 1·Zn2 are also shifted from 8.39 to 8.58 ppm upon addition of one equivalent of tris(tetrabutylammonium) hydrogen pyrophosphate (see ESI†). Intriguingly, significant shifts are also observed for the protons on the oxazole rings and the α-protons of the cyclic peptide, suggesting that either a change in peptide geometry occurs upon binding of PPi or that the amide protons in the cyclic peptide itself may provide a secondary binding site for PPi. This may explain the high affinity and selectivity that 1·Zn2 exhibits for PPi over similar anions. The complex retains C2 symmetry upon binding PPi, indicating that both zinc(II) ions are involved in the binding process (Fig. 2), but there are a number of binding modes that satisfy this requirement and we have not yet been able to determine the exact mode in which the PPi is bound by 1·Zn2.
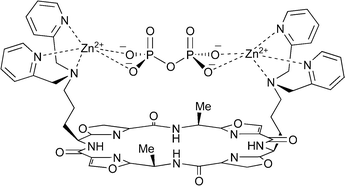 | ||
| Fig. 2 One of the possible binding modes for the 1·Zn2·PPi complex. | ||
In summary, a fluorescent chemosensing ensemble, based on a backbone modified cyclic peptide receptor and a coumarin indicator has been prepared. This ensemble detects PPi in water with a selectivity of two orders of magnitude over ADP and ATP and complete selectivity over monophosphate anions.
Notes and references
- W. N. Lipscomb and N. Sträter, Chem. Rev., 1996, 96, 2375 CrossRef CAS and references therein.
- Y. J. Jang, E. J. Jun, Y. J. Lee, Y. S. Kim, J. S. Kim and J. Yoon, J. Org. Chem., 2005, 70, 9603 CrossRef CAS; H. K. Cho, D. H. Lee and J.-I. Hong, Chem. Commun., 2005, 1690 RSC; D. H. Lee, S. Y. Kim and J.-I. Hong, Angew. Chem., Int. Ed., 2004, 43, 4777 CrossRef CAS; D. H. Lee, J. H. Im, S. U. Son, Y. K. Chung and J.-I. Hong, J. Am. Chem. Soc., 2003, 125, 7752 CrossRef CAS; S. Mizukami, T. Nagano, Y. Urano, A. Odani and K. Kikuchi, J. Am. Chem. Soc., 2002, 124, 3920 CrossRef CAS; L. Fabbrizzi, N. Marcotte, F. Stomeo and A. Taglietti, Angew. Chem., Int. Ed., 2002, 41, 3811 CrossRef CAS.
- R. G. Hanshaw, S. M. Hilkert, H. Jiang and B. D. Smith, Tetrahedron Lett., 2004, 45, 8721 CrossRef CAS.
- P. Wipf, in Alkaloids: Chemical and Biological Perspectives, ed. S. W. Pelletier, Elsevier, Amsterdam, 1998, vol. 12, ch. 3, pp. 187–228 Search PubMed.
- K. A. Jolliffe, Supramol. Chem., 2005, 17, 81 CrossRef CAS; A. J. Lucke, J. D. A. Tyndall, Y. Singh and D. P. Fairlie, J. Mol. Graphics Modell., 2003, 21, 341 CrossRef CAS; Y. Singh, N. Sokolenko, M. J. Kelso, L. R. Gahan, G. Abbenante and D. P. Fairlie, J. Am. Chem. Soc., 2001, 123, 333 CrossRef CAS; L. Somogyi, G. Haberhauer and J. Rebek, Jr., Tetrahedron, 2001, 57, 1699 CrossRef CAS; C. Boss, P. H. Rasmussen, A. R. Wartini and S. R. Waldvogel, Tetrahedron Lett., 2000, 41, 6327 CrossRef CAS; G. Haberhauer, L. Somogyi and J. Rebek, Jr., Tetrahedron Lett., 2000, 41, 5013 CrossRef CAS; D. Mink, S. Mecozzi and J. Rebek, Jr., Tetrahedron Lett., 1998, 39, 5709 CrossRef CAS.
- S. L. Wiskur, H. Ait-Haddou, J. J. Lavigne and E. V. Anslyn, Acc. Chem. Res., 2001, 34, 963 CrossRef CAS.
- M. S. Han and D. H. Kim, Angew. Chem., Int. Ed., 2002, 41, 3809 CrossRef CAS; A. Ojida, Y. Mito-oka, M. Inoue and I. Hamachi, J. Am. Chem. Soc., 2002, 124, 6256 CrossRef CAS; A. Ojida, M. Inoue, Y. Mito-oka and I. Hamachi, J. Am. Chem. Soc., 2003, 125, 10184 CrossRef CAS.
- A. Ojida, Y. Mito-oka, K. Sada and I. Hamachi, J. Am. Chem. Soc., 2004, 126, 2454 CrossRef CAS.
- The synthesis of 3 and related cyclic peptide platforms will be reported elsewhere.
- Synthesis and spectral data for 1: Compound 3 (100 mg, 0.11 mmol) was treated with HBr in acetic acid (33 wt%, 4 mL) for 30 min. The solution was then slowly added to a stirred solution of anhydrous Et2O (20 mL), resulting in the formation of a colourless precipitate which was collected by filtration. The precipitate was dissolved in MeOH (20 mL) and Amberlite IRA-400 resin (200 mg) was then added. Removal of the resin by filtration, followed by concentration of the filtrate, gave the crude free amine. This was suspended in CH2Cl2 (10 mL) and the suspension was treated with 2-pyridinecarboxyaldehyde (150 µL, 1.57 mmol) and sodium triacetoxyborohydride (510 mg, 2.4 mmol) for 4 h. More 2-pyridinecarboxyaldehyde (75 µL, 0.78 mmol) and sodium triacetoxyborohydride (255 mg, 1.2 mmol) were then added and the mixture was left to stir for 18 h. The resulting mixture was filtered through a plug of silica (EtOAc) and the filtrate concentrated. The residue was purified by flash chromatography (50 ∶ 50 ∶ 1 EtOAc–toluene–Et3N to 10 ∶ 90 ∶ 1 MeOH–EtOAc–Et3N) to yield (1) as a pale yellow oil (66 mg, 54%), [α]D = −86.8° (c 0.65, MeOH); 1H NMR (CD3OD/300 MHz) δ 1.60 (m, 4H), 1.67 (d, J 5.4 Hz, 6H), 1.92 (m, 4H), 2.06 (m, 4H), 2.60 (ABq, 4H), 3.74 (s, 8H), 5.28, (dd, J 6.4, 8.7 Hz, 4H), 5.48 (ABq, 4H), 7.25 (m, 4H), 7.62 (br d, J 7.8 Hz, 2H), 7.74 (m, 4H), 8.41 (s, 2H), 8.42 (s, 2H), 8.45 (br d, J 4.4 Hz, 4H). 13C NMR (75 MHz): δ 18.1, 23.4, 30.8, 42.5, 46.1, 53.9, 60.3, 122.8, 123.9, 135.8, 135.8, 137.6, 142.8, 143.0, 148.4, 159.6, 160.7, 161.0, 164.7, 165.1. MS (ESI) m/z 1025 ([M + Na]+, 100%); HRMS (ESI) calcd for C52H54N14O8 [M + Na]+ 1025.4147, found 1025.4142, calcd for C52H54N14O8 [M + 2Na]2+ 524.2022, found 524.2017.
- L. A. Morris, M. Jaspars, J. J. Kettenes-van den Bosch, K. Versluis, A. J. R. Heck, S. M. Kelly and N. C. Price, Tetrahedron, 2001, 57, 3185 CrossRef CAS; L. Grøndahl, N. Sokolenko, G. Abbenante, D. P. Fairlie, G. R. Hanson and L. R. Gahan, J. Chem. Soc., Dalton Trans., 1999, 1227 RSC; D. J. Freeman, G. Pattenden, A. F. Drake and G. Siligardi, J. Chem. Soc., Perkin Trans. 2, 1998, 129 RSC.
- R. G. Hanshaw, E. J. O'Neil, M. Foley, R. T. Carpenter and B. D. Smith, J. Mater. Chem., 2005, 15, 2707 RSC.
- K. A. Connors, Binding Constants, Measurement of Molecular Complex Stability, John Wiley and Sons, New York, 1987 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR spectra of 1, 1·Zn2 and the pyrophosphate complex; mass spectra and fluorescence data. See DOI: 10.1039/b606917g |
| ‡ Since the Dpa ligands do not saturate the binding sites of the Zn(II) ions, it is possible that O or N donors from the cyclic peptide occupy the other coordination sites. |
| § All anion binding experiments were also performed under these conditions. |
| This journal is © The Royal Society of Chemistry 2006 |
