Luminescent logic function of a surfactant-encapsulated polyoxometalate complex†
Hui
Zhang
,
Xiankun
Lin
,
Yi
Yan
and
Lixin
Wu
*
Key Laboratory for Supramolecular Structure and Materials of Ministry of Education, Jilin University, Changchun, China. E-mail: wulx@jlu.edu.cn; Fax: +86 431 5193421; Tel: +86 431 5168481
First published on 21st July 2006
Abstract
We have fabricated a novel organic/inorganic hybrid material consisting of multifunctional surfactant-encapsulated polyoxometalloeuropate which functions as a luminescent logic gate with dual output operated by light and metal ion as inputs.
Polyoxometalates (POMs) are discrete, molecular metal oxides with a wide variety of compositions, which exhibit an extensive range of chemical and electronic properties and have applications which include luminescent, electro-optical, magnetic and biological aspects.1 Recently, important progress has been made on the encapsulation of the POM with organic molecules using cooperative electrostatic interactions, forming multifunctional organic/inorganic hybrid materials.2–4 Cationic surfactants can replace the counterions of POMs, forming discrete surfactant-encapsulated polyoxometalate complexes (SECs), which not only improve the processibility of POMs but also provide a novel organic/inorganic hybrid building block for supramolecular assemblies. Some SECs have already been reported by our group with regard to the organization of layered and honeycomb structures, liquid crystal materials and luminescent properties in a polymer.4 The significant advantages of these complexes include small fixed dimensions of 3–5 nm, thermal and oxidative stability and their not suffering from the limitations of size and process reproducibility, thus making them particularly attractive for use as active “bottom-up” materials in molecular devices.5
Chemical computation by molecular devices is an ultimate challenge of future technology, which is envisioned to be able to break through the size limitation of current silicon-based microelectronic devices.6 Molecular logic gates such as AND,7a NOT,7b OR7c and their combinational logic circuits (e.g. NOR and INHIBIT)8 that generally produce light signals in response to a variety of inputs have been studied extensively. Recent interest is focused on an integrated system involving multiple fluorescent output modes.8–10 Therefore, providing more functional materials with excellent properties is an exigent challenge. Most present studies are focused on the synthesized organic molecules9 or DNA,10 however, little attention has been paid to hybrid materials. In this communication, by exploiting the advantage of the supramolecular self-assembly process, we first report a luminescent logic gate with dual output based on SEC realized by utilizing synergetic interaction between each component.
Fig. 1 indicates the novel design of a luminescent logic gate based on SEC consisting of two components connected together through electrostatic interaction. One is the luminescent polyoxometalloeuropate (Na9EuW10O36, POM-1). The photoexcitation of the oxygen-to-metal charge transfer (O→W LMCT) bands of the POM-1 leads to the luminescence of Eu3+ (5D0→7FJ, J = 0–4).3b,11 The other is a multi-functional surfactant, trans-10-(4-(4′-pyridylvinylene)-phenyl)oxydecyldodecyldimethylammonium bromide (PyC10C12N), which is used to encapsulate POM-1 through electrostatic interaction. The preparations of PyC10C12N and the surfactant-encapsulated POM-1 complex (SEC-1) were carried out according to the reported procedure2–4 and are presented in the supporting information. Furthermore, two different treatments can be applied to the terminal fluorescent stilbazole group of PyC10C12N, i.e. coordination of metal ions12 and trans–cis isomerization induced by UV irradiation,13 which can influence the fluorescence of the stilbazole group. Also, as demonstrated below, through supramolecular synergy between PyC10C12N and POM-1, the two treatments would also affect the fluorescence of POM-1, which might further result in a luminescent logic gate based on SEC-1 with metal ion and UV irradiation as inputs.
 | ||
| Fig. 1 Structural illustration of SEC-1. | ||
First, we studied the influence of the addition of metal ions on the luminescence of SEC-1. Fig. 2a shows the emission spectrum of SEC-1 in CH2Cl2 (3.24 × 10−5 M) excited at 260 nm. It indicates strong characteristic Eu3+ emissions at 590 nm (5D0→7F1) and 614 nm (5D0→7F2) which originate from the intramolecular energy transfer from the O→W LMCT excited states to the emissive state of Eu3+ (5D1 and 5D0)3b and a weak band at about 400 nm that is assigned to the characteristic emission of the stilbazole.12 Upon the addition of zinc ions, the Eu3+ emission bands are weakened and this is accompanied by the appearance of a new emission band with significantly increased intensity at 480 nm which originates from the zinc coordinated stilbazole (Fig. 2b). This treatment changes the luminescent color of the SEC-1 solution from red to white (excited by 254 nm light) as shown in the Fig. 2 inset. In contrast to the present case, the addition of zinc ions to the pure PyC10C12N without POM-1 does not induce significant fluorescent spectral changes (Fig. S3) and the slight red-shift with respect to the free ligand should be due to the perturbation of the electronic structure of PyC10C12N by nitrogen coordination, which is in accordance with the observation in the literature.12
 | ||
| Fig. 2 Fluorescent spectra (λex = 260 nm) of SEC-1 in CH2Cl2 under four different conditions: (a) neat SEC-1 solution; (b) after addition of ZnCl2; (c) after irradiation with 365 nm light for 3 min; (d) irradiation with 365 nm light after adding ZnCl2. Inset: Digital photographs at the corresponding conditions under illumination with 254 nm light. | ||
To explain this phenomenon, we carefully examined the spectral behavior of SEC-1 before and after adding ZnCl2. Fig. 3a shows the UV–vis spectrum of SEC-1 solution. The absorption band of POM-1 is located below 300 nm1d and the band at 328 nm can be assigned to π→π* transition of the stilbazole group of SEC-1 or its overlapping with n→π* transition due to the weak electron-donating ability of the alkoxy group.12 After adding ZnCl2, the absorption band of stilbazole is red-shifted and appears in the region of 270–410 nm (Fig. 3b), which overlaps the transition within the 4f6 shell of Eu3+ appearing at 390 nm,3b thus implying that the energy from the O→W LMCT excitation states of zinc coordinated SEC-1 will not completely transfer to the emissive state of Eu3+ as in the case of photoexcitation of POM-1, but partially transfers to the emissive state of zinc coordinated stilbazole. As a result, after coordination to zinc ion, the emission intensity of Eu3+ in SEC-1 is weakened and that of zinc coordinated stilbazole is enhanced.
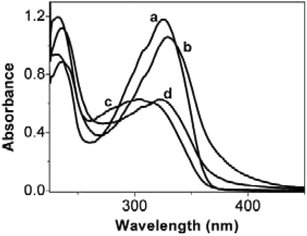 | ||
| Fig. 3 UV–vis spectra of SEC-1 in CH2Cl2 under four different conditions: (a) neat SEC-1 solution; (b) after addition of ZnCl2; (c) after irradiation with 365 nm light for 3 min; (d) irradiation with 365 nm light after adding ZnCl2. | ||
Second, we studied the effect of the photoinduced trans–cis isomerization of the stilbazole group on the luminescence of SEC-1. On irradiating the solution of SEC-1 with 365 nm light for 3 min, the trans-stilbazole is converted to cis-isomer (Fig. 3a and 3c), which induces remarkable fluorescent spectral changes of SEC-1. The emission intensity of stilbazole (at about 400 nm) is decreased and that of the POM-1 is also weakened (Fig. 2c). The former change is derived from the fact that the cis-isomer of the stilbazole group is nonfluorescent in solution.13 The latter change may be because the excitation band of the POM-1 (O→W LMCT band, appearing at 240–310 nm, Fig. S1) and the absorption band of the cis-isomer of stilbazole (below 280 nm) overlap, which might lead to the formation of a non-fluorescent complex.14 The mechanism is currently under investigation.
Based on the above-mentioned principles, when adding ZnCl2 and applying UV light irradiation (365 nm) simultaneously (Fig. 3d), the emission intensity of the zinc coordinated stilbazole of SEC-1 is significantly reduced and the emission of Eu3+ is greatly suppressed (Fig. 2d).
Furthermore, we demonstrate the logic operation of SEC-1 with metal ions (Input1) and UV irradiation (365 nm) (Input2) as inputs, two fluorescence bands monitored at 480 nm (Output1) and 614 nm (Output2) as outputs. To indicate clearly the logic function of SEC-1 as a logic gate, the four situations (Fig. 2 and Fig. 4) are examined. The fluorescent intensity at 480 nm (Output1) is high only when UV irradiation is absent and in the presence of Zn2+, expressing the INHIBIT logic function. The fluorescent intensity at 614 nm (Output2) is high only when both UV irradiation and Zn2+ are absent, expressing the NOR logic function. Therefore, the system operates as a luminescent logic gate with dual output. The corresponding truth table and logic scheme are shown in Fig. 4.
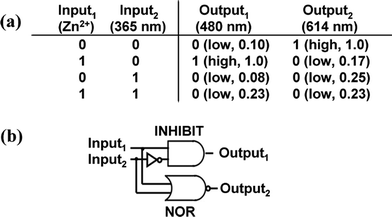 | ||
| Fig. 4 Truth table (a) and logic scheme (b) of the logic gate based on SEC-1. | ||
In conclusion, we have utilized a supramolecular self-assembly method to fabricate a new organic/inorganic hybrid material consisting of SEC-1 with its dual output logic function (INHIBIT and NOR) realized by the supramolecular synergy between two components. The present methodology indicates that, besides organic molecules, the inorganic nanosized materials can be introduced into the logic gate as well. Due to the small fixed size and the structural, chemical and electronic versatility of POM clusters, the introduction of POMs may create an opportunity for the further development of the logic gate system. Moreover, the supramolecular self-assembly process allows easy development of other functional logic gates by altering each component in the hybrid (either the inorganic or the organic part), thereby providing a general design platform for the logic gate and other molecular devices composed of organic/inorganic hybrid materials.
This work is financially supported by the National Natural Science Foundation of China (Grant No. 20473032 and 20574030), PCSIRT of the Ministry of Education of China (IRT0422), the Innovation Fund of Jilin University and the Open Project of the State Key Laboratory of Polymer Physics and Chemistry of CAS.
Notes and references
- (a) C. L. Hill, Chem. Rev., 1998, 98, 1–390 CrossRef CAS (the entire issue is devoted to polyoxometalates); (b) E. Coronado and C. Mingotaud, Adv. Mater., 1999, 11, 869 CrossRef CAS; (c) N. Casañ-Pastor and P. Gómez-Romero, Front. Biosci., 2004, 9, 1759 Search PubMed; (d) G. Zhang, Z. Chen, T. He, H. Ke, Y. Ma, K. Shao, W. Yang and J. Yao, J. Phys. Chem. B, 2004, 108, 6944 CrossRef CAS.
- S. Polarz, B. Smarsly and M. Antonietti, ChemPhysChem, 2001, 7, 457 CrossRef; D. G. Kurth, P. Lehmann, D. Volkmer, A. Müller and D. Schwahn, J. Chem. Soc., Dalton Trans., 2000, 3989 RSC.
- (a) W. Bu, L. Wu, X. Zhang and A.-C. Tang, J. Phys. Chem. B, 2003, 107, 13425 CrossRef CAS; (b) W. Bu, H. Li, W. Li, L. Wu, C. Zhai and Y. Wu, J. Phys. Chem. B, 2004, 108, 12776 CrossRef CAS.
- (a) W. Bu, H. Li, H. Sun, S. Yin and L. Wu, J. Am. Chem. Soc., 2005, 127, 8016 CrossRef CAS; (b) W. Li, W. Bu, H. Li, L. Wu and M. Li, Chem. Commun., 2005, 3785 RSC; (c) H. Li, W. Qi, W. Li, H. Sun and L. Wu, Adv. Mater., 2005, 17, 2688 CrossRef CAS.
- E. Kapetanakis, P. Normand and D. Tsoukalas, Appl. Phys. Lett., 2002, 80, 2794 CrossRef CAS; N. Glezos, P. Argitis, D. Velessiotis and C. D. Diakoumakos, Appl. Phys. Lett., 2003, 83, 488 CrossRef CAS.
- R. Ballardini, V. Balzani, A. Credi, M. T. Gandolfi and M. Venturi, Acc. Chem. Res., 2001, 34, 445 CrossRef CAS.
- (a) A. P. de Silva, H. Q. N. Gunaratne and C. P. McCoy, Nature, 1993, 364, 42 CrossRef; (b) A. P. de Silva, A. S. Dissanayake and K. R. A. S. Sandanayake, J. Chem. Soc., Chem. Commun., 1989, 1054 RSC; (c) A. P. de Silva, H. Q. N. Gunaratne and G. E. M. Maguire, J. Chem. Soc., Chem. Commun., 1994, 1213 RSC.
- F. M. Raymo, Adv. Mater., 2002, 14, 401 CrossRef CAS; A. P. de Silva and N. D. McClenaghan, Chem.–Eur. J., 2004, 10, 574 CrossRef.
- D. Margulies, G. Melman, C. E. Felder, R. Arad-Yellin and A. Shanzer, J. Am. Chem. Soc., 2004, 126, 15400 CrossRef CAS; Y. Shiraishi, Y. Tokitoh and T. Hirai, Chem. Commun., 2005, 5316 RSC; A. Coskun, E. Deniz and E. U. Akkaya, Org. Lett., 2005, 7, 5187 CrossRef CAS.
- A. Saghatelian, N. H. Völcker, K. M. Guckian, V. S.-Y. Lin and M. R. Ghadiri, J. Am. Chem. Soc., 2003, 125, 346 CrossRef CAS; Y. Weizmann, R. Elnathan, O. Lioubashevski and I. Willner, J. Am. Chem. Soc., 2005, 127, 12666 CrossRef CAS.
- R. Ballardini, Q. G. Mulazzani, M. Venturl, F. Bolletta and V. Balzani, Inorg. Chem., 1984, 23, 300 CrossRef CAS; T. Yamase, T. Kobayashi, M. Sugeta and H. Naruke, J. Phys. Chem. A, 1997, 101, 5046 CrossRef CAS.
- D. G. Whitten and M. T. McCall, J. Am. Chem. Soc., 1969, 91, 5097 CrossRef CAS; E. Lucenti, E. Cariati, C. Dragonetti, L. Manassero and F. Tessore, Organometallics, 2004, 23, 687 CrossRef CAS; Y. V. Fedorov, O. A. Fedorova, E. N. Andryukhina, N. E. Shepel, M. M. Mashura, S. P. Gromov, L. G. Kuzmina, A. V. Churakov, J. A. K. Howard, E. Marmois, J. Oberlé, G. Jonsaukas and M. V. Alfimov, J. Phys. Org. Chem., 2005, 18, 1032 CrossRef CAS.
- D. H. Waldeck, Chem. Rev., 1991, 91, 415 CrossRef CAS; F. D. Lewis and X. Liu, J. Am. Chem. Soc., 1999, 121, 11928 CrossRef CAS.
- M. K. Johansson, H. Fidder, D. Dick and R. M. Cook, J. Am. Chem. Soc., 2002, 124, 6950 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthesis and characterization of PyC10C12N and SEC-1. See DOI: 10.1039/b606343h |
| This journal is © The Royal Society of Chemistry 2006 |
