Reading the operation of an acid/base-controllable molecular switch by naked eye†
Kuang-Wei
Cheng
a,
Chien-Chen
Lai
b,
Pinn-Tsong
Chiang
a and
Sheng-Hsien
Chiu
*a
aDepartment of Chemistry, National Taiwan University, Taipei, Taiwan 10617, R.O.C. E-mail: shchiu@ntu.edu.tw; Fax: +886 2 33661677; Tel: +886 2 33661675
bInstitute of Molecular Biology, National Chung Hsing University and Department of Medical Genetics, China Medical University Hospital, Taichung, Taiwan, R.O.C.
First published on 7th June 2006
Abstract
We report a pH-controllable molecular switch whose switching can be monitored by the naked eye; this system involves the formation of a complex between a [2]rotaxane—featuring dibenzylammonium and 4,4′-bipyridinium stations—and a TTF-side-walled molecular clip.
The synthesis of molecular switches and actuators that function through host–guest recognition continues to attract much attention because these systems have potential applicability in mesoscale molecular electronic devices.1 Because of the need for reversibility in the operation of these machine-like molecules, chemically controllable molecular switches are generally operated through the sequential addition and removal of electrons,2 metal ions,3 or protons.4 Among these systems, acid/base-controllable molecular switches are relatively simple to design because in general they operate through charge repulsion or hydrogen bonding interactions upon protonation of a receptor in the molecular structure of either the host or guest.4 One example of such a system is a [2]rotaxane in which the macrocyclic component can reside at one of two stations. Ideally, the binding affinity of this macrocycle should favor one station exclusively in the “on” state and the other station exclusively in the “off” state—with clear optical output signals in both cases—to ensure that the switching event can be monitored effectively. Most acid/base-controllable molecular switches do not, however, provide significant optical outputs during their switching; rather, their operating states are monitored using NMR spectroscopic techniques. Herein, we describe a pH-controllable molecular switch whose switching can be monitored by the naked eye; this system involves the formation of a complex between a [2]rotaxane—comprising macrocycle 1 and a dumbbell featuring dibenzylammonium and 4,4′-bipyridinium stations—and a TTF-side-walled molecular clip.5
The dual-use “oxygen-deficient” macrocycle 1 is the smallest known macrocycle that is capable of forming relatively strong complexes in solution with dibenzylammonium (DBA+) and bipyridinium ions.6 Because the binding affinity of 1 with DBA·PF6 (Ka = 200 ± 30 M−1) in CD3CN is ca. 10-fold higher than that with N,N′-dimethylbipyridinium bishexafluorophosphate (Ka = 26 ± 2 M−1), we suspected that a two-station [2]rotaxane comprising these units would operate as an acid/base-controllable molecular switch. We synthesized (Scheme 1) such a [2]rotaxane through the reaction of the dibenzylammonium ion 2-H·PF67 with the monopyridinium salt 3·PF68 in CH3CN in the presence of macrocycle 1; the semirotaxane9 intermediate [1⊃2-H][PF6] reacted with 3·PF6 to afford the [2]rotaxane 4-H·3PF6 in 35% yield after subsequent ion exchange and purification processes.
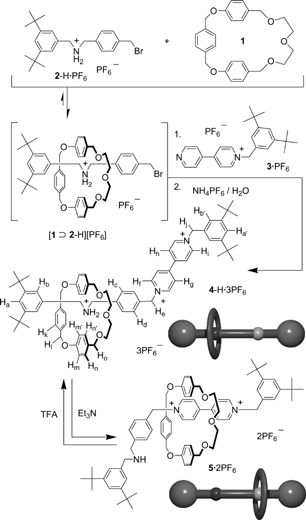 | ||
| Scheme 1 | ||
The 1H NMR spectrum of rotaxane 4-H·3PF6 displays (Fig. 1) significant upfield shifts in the signals of the methylene protons adjacent to the ammonium center relative to those of the free dibenzylammonium ion 2-H·PF6, which suggests that the macrocyclic unit encircles the CH2NH2+CH2 center.10 Because the association and dissociation processes of the dibenzylammonium ion and macrocycle 1 are slow on the 1H NMR spectroscopic timescale,11 if macrocycle 1 were to reside anywhere else along the dumbbell-shaped component, i.e., other than on the NH2+ center, we should observe another set of signals representing a second translational isomer; because we do not, it appears that the macrocyclic unit in 4-H·3PF6 resides at the NH2+ center with good selectivity in CD3CN at room temperature. The addition of Et3N to this solution led to deprotonation of the NH2+ center, which resulted in the disappearance of the multiplet in the 1H NMR spectrum that we assign to its adjacent methylene protons (Fig. 1b). Meanwhile, the signals for the α and β protons of the bipyridinium unit underwent upfield shifts, implying the formation of the [2]rotaxane 5·2PF6, in which the macrocyclic unit encircles the bipyridinium unit. Subsequent addition of trifluoroacetic acid (TFA) provided a 1H NMR spectrum (Fig. 1c) similar to that of the original solution of rotaxane 4-H·3PF6 (Fig. 1a); this observation implies that the macrocyclic unit had migrated back to the NH2+ center to regenerate the original rotaxane 4-H+ in solution.
![Partial 1H NMR spectra [400 MHz, CD3CN/CDCl3 (5 ∶ 1), 298 K] of (a) rotaxane 4-H·3PF6, (b) the mixture obtained after adding Et3N (2 equiv) to the solution in (a), and (c) the mixture obtained after adding TFA (2 equiv) to the solution in (b).](/image/article/2006/CC/b606203b/b606203b-f1.gif) | ||
| Fig. 1 Partial 1H NMR spectra [400 MHz, CD3CN/CDCl3 (5 ∶ 1), 298 K] of (a) rotaxane 4-H·3PF6, (b) the mixture obtained after adding Et3N (2 equiv) to the solution in (a), and (c) the mixture obtained after adding TFA (2 equiv) to the solution in (b). | ||
Although the switching of the macrocyclic unit from the NH2+ unit to the bipyridinium moiety upon the addition of Et3N did induce a weak charge transfer absorption between the electron-rich phenol ring and the electron-deficient bipyridinium unit—turning the solution from colorless to light yellow—this transformation was not very striking to the naked eye, especially at low concentration. Previously, we demonstrated that the TTF-side-walled molecular clip 6 forms a complex with N,N′-dimethylbipyridinium bishexafluorophosphate in CD3CN (Ka = 5600 ± 300 M−1);12 this complex displays a green color that arises from a charge transfer absorption between the bipyridinium ion and the TTF units.13 Thus, we suspected that mixing the molecular clip 6 with the [2]rotaxane 4-H·3PF6 in CD3CN would lead to the formation of a [2]rotaxane/molecular clip complex (6⊃4-H·3PF6) in which the molecular clip coordinates to the free bipyridinium unit to result in a green solution (Scheme 2). The subsequent addition of Et3N to this solution was expected to cause the macrocyclic unit of the [2]rotaxane to compete with the molecular clip for the bipyridinium center; ideally, this process would lead to dissociation of the complex to provide the free [2]rotaxane 5·2PF6 and the free molecular clip 6 with simultaneous disappearance of the green color.
 | ||
| Scheme 2 | ||
Gratifyingly, solutions of the colorless [2]rotaxane 4-H·3PF6 and the yellow molecular clip formed a green solution upon their mixing (Fig. 2A-a). The UV-Vis spectrum (Fig. 2B-a) of the complex formed between 4-H·3PF6 and 6 in MeCN/CHCl3 (5 ∶ 1) displays a charge transfer band at 709 nm. The 1H NMR spectrum of this solution indicates (Fig. 3b) that upfield shifts occurred to the signals of the β-pyridinium protons of the [2]rotaxane and the PhOCH2 protons of the molecular clip; these changes are similar to those that occur after the complexation of molecular clip 6 with N,N′-dimethylbipyridinium bishexafluorophosphate under similar conditions.11 A Job plot based on the signal of the PhOCH2 protons in the 1H NMR spectrum of the molecular clip afforded conclusive evidence (see Supporting Information) for 1 ∶ 1 complexation in CD3CN/CDCl3 (5 ∶ 1). The electrospray ionization (ESI) mass spectrum recorded on an equimolar mixture of 6 and 4-H·3PF6 reveals two peaks at m/z 1374 and 867, which correspond to the formation of complexes [6⊃4-H·PF6]2+ and [6⊃4-H]3+, respectively. These results suggest the formation of the [2]rotaxane/clip complex (6⊃4-H)·3PF6, for which we determined the association constant (Ka) in CD3CN/CDCl3 (5 ∶ 1) to be 4100 ± 400 M−1, based on a 1H NMR spectroscopic dilution experiment.14 The addition of Et3N to the mixture immediately switched the color of the solution back to yellow, with a concomitant substantial decrease in the intensity of the charge transfer absorption in the UV spectrum (cf.Figs. 2A-b and 2B-b); in addition, the corresponding 1H NMR spectrum displays the existence of both the free molecular clip 6 and [2]rotaxane 5·2PF6 in the solution (Fig. 3c). Thus, the [2]rotaxane/molecular clip complex dissociated upon the addition of Et3N, most likely as a result of the macrocyclic unit encircling the bipyridinium station in 5·2PF6 to the exclusion of the molecular clip 6. Subsequent addition of TFA to this solution restored the charge transfer band at 709 nm (Fig. 2B-c), immediately switched the color of the solution back to green (Fig. 2A-c), and resulted in a 1H NMR spectrum (Fig. 3d) similar to that of the mixture of only molecular clip 6 and [2]rotaxane 4-H·3PF6 (Fig. 3b); this evidence suggests that regeneration of the [2]rotaxane/molecular clip complex occurred in solution upon protonation of the amino center in 5·2PF6. Thus, sequential addition of a base and an acid induces migration of the macrocyclic unit of this [2]rotaxane from the NH2+ station to the bipyridinium unit and back again, in turn controlling the availability of the bipyridinium station for complexation with the molecular clip 6 and resulting in significant color changes that are clearly visible to the naked eye. This process occurs without the need to covalently link chromophore(s) to the macrocyclic and/or dumbbell-shaped components.
![(A) Photograph depicting the color changes that occur to the solution [MeCN/CHCl3 (5 ∶ 1), 4 mM, 298 K] during the switching process and (B) the corresponding partial UV-Vis spectra [MeCN/CHCl3 (5 ∶ 1), 2 mM, 298 K] of (a) an equimolar mixture of 4-H·3PF6 and 6, (b) the mixture obtained after adding Et3N (2 equiv) to solution (a), and (c) the mixture obtained after adding TFA (2 equiv) to solution (b).](/image/article/2006/CC/b606203b/b606203b-f2.gif) | ||
| Fig. 2 (A) Photograph depicting the color changes that occur to the solution [MeCN/CHCl3 (5 ∶ 1), 4 mM, 298 K] during the switching process and (B) the corresponding partial UV-Vis spectra [MeCN/CHCl3 (5 ∶ 1), 2 mM, 298 K] of (a) an equimolar mixture of 4-H·3PF6 and 6, (b) the mixture obtained after adding Et3N (2 equiv) to solution (a), and (c) the mixture obtained after adding TFA (2 equiv) to solution (b). | ||
![Partial 1H NMR spectra [400 MHz, CD3CN/CDCl3 (5 ∶ 1), 298 K] of (a) rotaxane 4-H·3PF6, (b) an equimolar (10 mM) mixture of rotaxane 4-H·3PF6 and molecular clip 6, (c) the mixture obtained after adding Et3N (4 equiv) to the solution in (b), and (d) the mixture obtained after adding TFA (4 equiv) to the solution in (c).](/image/article/2006/CC/b606203b/b606203b-f3.gif) | ||
| Fig. 3 Partial 1H NMR spectra [400 MHz, CD3CN/CDCl3 (5 ∶ 1), 298 K] of (a) rotaxane 4-H·3PF6, (b) an equimolar (10 mM) mixture of rotaxane 4-H·3PF6 and molecular clip 6, (c) the mixture obtained after adding Et3N (4 equiv) to the solution in (b), and (d) the mixture obtained after adding TFA (4 equiv) to the solution in (c). | ||
We have demonstrated that the presence of a TTF-side-walled molecular clip allows the mechanical motion of a pH-controllable molecular switch to be monitored by the naked eye. We believe that such rotaxane/molecular clip complexes may play roles in functional chemically, electrochemically, and photochemically controllable molecular switches, and possibly in new types of “supramolecular nanovalves”15 that control the flow of important molecules within porous materials.
Notes and references
- (a) Molecular Electronics: Science and Technology; A. Aviram and M. Ratner, Eds.; New York Academy of Sciences: New York, 1998 Search PubMed; (b) C. P. Collier, G. Mattersteig, E. W. Wong, Y. Luo, K. Beverly, J. Sampaio, F. M. Raymo, J. F. Stoddart and J. R. Heath, Science, 2000, 289, 1172 CrossRef CAS; (c) H. Yu, Y. Luo, K. Beverly, J. F. Stoddart, H.-R. Tseng and J. R. Health, Angew. Chem., Int. Ed., 2003, 42, 5706 CrossRef CAS.
- (a) A. Mirzoian and A. E. Kaifer, Chem.–Eur. J., 1997, 3, 1052 CrossRef CAS; (b) N. Armaroli, V. Balzani, J.-P. Collin, P. Gaviña, J.-P. Sauvage and B. Ventura, J. Am. Chem. Soc., 1999, 121, 4397 CrossRef CAS; (c) A. Altieri, F. G. Gatti, E. R. Kay, D. A. Leigh, D. Martel, F. Paolucci, A. M. Z. Slawin and J. K. Y. Wong, J. Am. Chem. Soc., 2003, 125, 8644 CrossRef CAS; (d) W. S. Jeon, A. Y. Ziganshina, J. W. Lee, Y. H. Ko, J.-K. Kang, C. Lee and K. Kim, Angew. Chem., Int. Ed., 2003, 42, 4097 CrossRef CAS; (e) H.-R. Tseng, S. A. Vignon, P. C. Celestre, J. Perkins, J. O. Jeppesen, A. Di Fabio, R. Ballardini, M. T. Gandolfi, M. Venturi, V. Balzani and J. F. Stoddart, Chem.–Eur. J., 2004, 10, 155 CrossRef CAS.
- (a) J.-P. Collin, C. Dietrich-Buchecker, P. Gavina, M. C. Jimenez-Molero and J.-P. Sauvage, Acc. Chem. Res., 2001, 34, 477 CrossRef CAS; (b) G. Kaiser, T. Jarrosson, S. Otto, Y.-F. Ng, A. D. Bond and J. K. M. Sanders, Angew. Chem., Int. Ed., 2004, 43, 1959 CrossRef CAS; (c) T. Iijima, S. A. Vignon, H.-R. Tseng, T. Jarrosson, J. K. M. Sanders, F. Marchioni, M. Venturi, E. Apostoli, V. Balzani and J. F. Stoddart, Chem.–Eur. J., 2004, 10, 6375 CrossRef.
- (a) A. M. Elizarov, S.-H. Chiu and J. F. Stoddart, J. Org. Chem., 2002, 67, 9175 CrossRef CAS; (b) J. W. Lee, K. Kim and K. Kim, Chem. Commun., 2001, 1042 RSC; (c) M.-V. Martínez-Díaz, N. Spencer and J. F. Stoddart, Angew. Chem., Int. Ed. Engl., 1997, 36, 1904 CrossRef CAS; (d) J. D. Badjic', V. Balzani, A. Credi, S. Silvi and J. F. Stoddart, Science, 2004, 303, 1845 CrossRef CAS; (e) F. Huang, K. A. Switek and H. W. Gibson, Chem. Commun., 2005, 3655 RSC.
- For glycoluril-based molecular clips, see: (a) A. E. Rowan, J. A. A. W. Elemans and R. J. M. Nolte, Acc. Chem. Res., 1999, 32, 995 CrossRef CAS; (b) J. N. H. Reek, J. A. A. W. Elemans, R. de Gelder, P. T. Beurskens, A. E. Rowan and R. J. M. Nolte, Tetrahedron, 2003, 59, 175 CrossRef CAS; (c) A. Wu, A. Chakraborty, J. C. Fettinger, R. A. Flowers, II and L. Isaacs, Angew. Chem., Int. Ed., 2002, 41, 4028 CrossRef CAS; (d) A. Wu, P. Mukhopadhyay, A. Chakraborty, J. C. Fettinger and L. Isaacs, J. Am. Chem. Soc., 2004, 126, 10035 CrossRef CAS; (e) P.-N. Cheng, P.-T. Chiang and S.-H. Chiu, Chem. Commun., 2005, 1285 RSC.
- P.-N. Cheng, P.-Y. Huang, W.-S. Li, S.-H. Ueng, W.-C. Hung, Y.-H. Liu, C.-C. Lai, S.-M. Peng, I. Chao and S.-H. Chiu, J. Org. Chem., 2006, 71, 2373 CrossRef CAS.
- S. J. Rowan, S. J. Cantrill, J. F. Stoddart, A. J. P. White and D. J. Williams, Org. Lett., 2000, 2, 759 CrossRef CAS.
- P. R. Ashton, R. Ballardini, V. Balzani, I. Baxter, A. Credi, M. C. T. Fyfe, M. T. Gandolfi, M. Gomez-Lopez, M.-V. Martínez-Díaz, A. Piersanti, N. Spencer, J. F. Stoddart, M. Venturi, A. J. P. White and D. J. Williams, J. Am. Chem. Soc., 1998, 120, 11932 CrossRef CAS.
- A semirotaxane has been defined as a pseudorotaxane possessing a stoppering unit at one terminus of the rod-like component. See: (a) J.-P. Collin, P. Gaviña and J.-P. Sauvage, New J. Chem., 1997, 21, 525 Search PubMed; (b) C. Seel, A. H. Parham, O. Safarowsky, G. M. Hübner and F. Vögtle, J. Org. Chem., 1999, 64, 7236 CrossRef CAS.
- P.-N. Cheng, W.-C. Hung and S.-H. Chiu, Tetrahedron Lett., 2005, 46, 4239 CrossRef CAS.
- (a) P. R. Ashton, P. J. Campbell, E. J. T. Chrystal, P. T. Glink, S. Menzer, D. Philp, N. Spencer, J. F. Stoddart, P. A. Tasker and D. J. Williams, Angew. Chem., Int. Ed. Engl., 1995, 34, 1865 CrossRef CAS; (b) P. R. Ashton, E. J. T. Chrystal, P. T. Glink, S. Menzer, C. Schiavo, N. Spencer, J. F. Stoddart, P. A. Tasker, A. J. P. White and D. J. Williams, Chem.–Eur. J., 1996, 2, 709 CrossRef CAS.
- P.-T. Chiang, P.-N. Cheng, C.-F. Lin, Y.-H. Liu, C.-C. Lai, S.-M. Peng and S.-H. Chiu, Chem.–Eur. J., 2006, 12, 865 CrossRef CAS.
- The molecules formed between a tetracationic cyclophane and a threaded TTF motif also display a green color; see: (a) B. W. Laursen, S. Nygaard, J. O. Jeppesen and J. F. Stoddart, Org. Lett., 2004, 6, 4167 CrossRef CAS; (b) W.-Q. Deng, A. H. Flood, J. F. Stoddart and W. A. Goddard, III, J. Am. Chem. Soc., 2005, 127, 15994 CrossRef CAS.
- K. A. Connors, Binding Constants; Wiley: New York, 1987 Search PubMed.
- (a) R. Hernandez, H.-R. Tseng, J. W. Wong, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2004, 126, 3370 CrossRef CAS; (b) T. D. Nguyen, H.-R. Tseng, P. C. Celestre, A. H. Flood, Y. Liu, J. F. Stoddart and J. I. Zink, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10029 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures for the preparation of 4-H·3PF6 and its characterization data. See DOI: 10.1039/b606203b |
| This journal is © The Royal Society of Chemistry 2006 |
