Selective holographic detection of glucose using tertiary amines
Kathryn E. S.
Dean
,
Adrian M.
Horgan
,
Alexander J.
Marshall
,
Satyamoorthy
Kabilan
and
John
Pritchard
*
Smart Holograms Ltd, 291 Cambridge Science Park, Milton Road, Cambridge, UK. E-mail: john.pritchard@smartholograms.co.uk; Fax: 01223 393401; Tel: 01223 393408
First published on 14th June 2006
Abstract
Introducing tertiary amine monomers into holographic sensors containing phenylboronic acids gives greatly improved selectivity for glucose.
To address the need for simple, robust and inexpensive analytical devices within the environmental, chemical, food and biomedical industries novel sensing systems based on ‘smart’ holograms are being developed.1–7 The holograms utilise the principles of volume holography with a Denisyuk holographic grating recorded within a ‘smart’ polymer. Unlike conventional holographic recording media, the ‘smart’ polymers contain receptors that allow the holograms to act as transducers. Interaction with a specific analyte changes the colour, image or brightness of the hologram and these changes can be easily visualised and quantified.
One analyte of significant interest is glucose. With the increasing prevalence of diabetes there is a real need for improved methods of glucose monitoring. Current electrochemical methods relying on enzymes are painful, inconvenient and provide only intermittent measurements, factors which may lead to reduced patient compliance and poorer glycaemic control. This can have potentially serious consequences as inadequate control over blood sugar increases the risk of developing diabetes-related complications such as heart disease, kidney disease and blindness.8
A different approach to glucose monitoring relies on a group of synthetic receptors known as phenylboronic acids. These Lewis acids can reversibly bind the cis-1,2- or -1,3-diols of saccharides covalently to form five- or six-membered rings.9 Unlike enzymes, phenylboronic acids have the advantage of being highly stable and able to resist moist heat, allowing them to be sterilized then used in vivo for continuous monitoring of glucose.
The ability of a biosensor to measure glucose accurately against a variable background of potential interferents is an essential requirement for real applications. Physiological fluids are composed of numerous substances which may potentially interact with the sensing element. The most likely interferents for biosensors based on phenylboronic acids are other saccharides possessing cis-1,2- or -1,3-diols. In healthy individuals glucose is normally present in the range 4–8 mM while fructose and galactose, the most abundant sugars after glucose, are usually present in physiological fluids at sub-millimolar levels.10 Unfortunately as Fig. 1 demonstrates, phenylboronic acids have a much greater affinity for fructose than glucose,11 a feature that may affect the accuracy of any glucose measurement. The increase in the diffraction peak wavelength (red-shift) with increasing sugar concentration in phosphate buffered saline (PBS, pH 7.4, I ∼ 150 mM) is due to volumetric expansion of the hydrogel and an increase in the spacing between the silver fringes of the holographic grating. These fringes selectively reflect light of a narrow band of wavelengths, reflecting longer wavelengths as their separation increases.† The increase in the volume of the hydrogel is attributed to the formation of charged boronate groups on binding cis-diols and the generation of a Donnan potential, causing the osmotic flow of water into the polymer. The much greater sensitivity of 3-acrylamidophenylboronic acid (3-APB) holograms to fructose than glucose poses a problem for blood glucose monitoring in patients with diabetes. Though the body is unable to use fructose without first converting it to glucose or glycogen in the liver, its presence in the blood and interaction with the phenylboronic acid biosensor potentially may lead to an artificially elevated glucose reading. This could result in no preventative clinical action being taken or the opposite treatment to that required with the associated risk of a potentially fatal episode of hypoglycaemia.
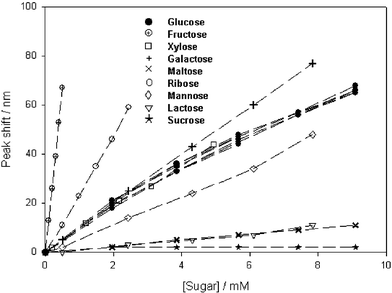 | ||
| Fig. 1 The shift in the peak diffraction wavelength of a 3 mol% MBA crosslinker, 12 mol% 3-APB receptor polyacrylamide hologram in response to different sugars (for glucose N = 8, for the other sugars N = 1). | ||
A strategy that has been employed for improving the selectivity of boronic acids relies on the observation that some monosaccharides possess more than one potential binding site. Thus, by altering the structure of the receptor so that the boronic acids are in favourable positions and orientations, one saccharide may be preferred over another.12 Rather than alter the chemical structure of the phenylboronic acid, the alternative strategy employed here is to tune the composition of the polymer. As Fig. 2 shows there is a dramatic change in behaviour when the tertiary amine monomer, N-[3-(dimethylamino)propyl]-acrylamide (DAPA), is incorporated into polyacrylamide hydrogel films containing 3-APB receptors. Instead of glucose causing swelling, there is now a glucose-specific blue-shift in the diffraction peak wavelength corresponding to volumetric contraction of the film perpendicular to the fringe planes. The most probable explanation for the observed contraction is crosslinking of two neighbouring boronic acid receptors with favourable stereochemistry by glucose to give a bis-boronate-glucose complex. Such complexes have previously been reported in the literature for fluorescent molecular chemosensors.13
 | ||
| Fig. 2 The shift in the peak diffraction wavelength of a 3 mol% MBA crosslinker, 12 mol% 3-APB receptor, 16 mol% DAPA tertiary amine polyacrylamide hologram in response to different sugars (for glucose N = 8, for the other sugars N = 1). | ||
The importance of the tertiary amine in promoting hydrogel contraction can be seen from experiments where the concentrations of receptor (3-APB) and tertiary amine (DAPA) are varied (results not shown). There is an increased response to both glucose (contraction) and fructose (swelling) on increasing the concentration of both monomers. This is because introducing more 3-APB into the hydrogel results in more saccharide binding sites, thus increasing the potential for hydrogel swelling. At the same time, increasing the number of adjacent tertiary amines increases the likelihood of contraction through crosslinking of those sites by glucose. At lower molar ratios of DAPA to 3-APB (∼0.3 ∶ 1, ∼0.7 ∶ 1) the potential for hydrogel swelling is greater while conversely at higher ratios, the likelihood of glucose induced hydrogel contraction increases. One explanation for the ability of the tertiary amines to induce glucose-specific crosslinking is their ability to form B–N type bonds with phenylboronic acids.14,15 Acting as Lewis bases, the tertiary amines may donate their lone pairs either directly to the boron atom or to a hydrogen of a solvent molecule coordinated to the boron atom. This may affect a change in the hybridisation state of nearby boron atoms from sp2 to sp3, which has the effect of lowering the pKa of the boronic acid. Once in the more reactive tetrahedral configuration, the boronic acid is better able to bind cis-diols due to improved overlap of the bonding orbitals leading to more stable ring structures. As noted for molecular chemosensors, glucose is relatively unusual in that it may bind two boronic acids simultaneously via two pairs of diols located at either end of the glucose molecule.12,13 It is likely that free radical polymerisation of DAPA with 3-APB, in the presence of N,N′-methylenebisacrylamide (MBA) crosslinker, results in the tertiary amines and boronic acids being in sufficient proximity within the hydrogel to allow bond formation to occur while at the same time placing boronic acids close enough for there to be crosslinking by glucose. As the concentrations of 3-APB and DAPA increase, the amines and boronic acids get closer and closer together increasing the potential for crosslinking.
To see if the contraction of the hydrogel in response to glucose would lead to an improvement in selectivity, the 3 mol% MBA, 12 mol% 3-APB, 16 mol% DAPA hologram shown in Fig. 2 was titrated with glucose in the presence of a very high background (0.1 mM) of fructose and the perturbation on the response of the sensor examined (results not shown). Newer, more accurate methods of analysis, which are not prone to interference from glucose, have determined serum fructose levels for healthy subjects and diabetics to be in fact much lower ∼8–12 µM.16,17 The perturbation to the sensor with a background of 0.1 mM fructose was minimal being within the error of the measurement. At even higher concentrations of fructose there was a slight decrease in the degree of contraction due to competition from fructose for the receptor binding sites. The latter observation emphasizes an advantage of relying on crosslinking and contraction of the hologram for glucose detection. In this case, the presence of fructose leads to a lower concentration of glucose being reported than actually present, thereby providing an early warning of hypoglycaemia. Thus, the fructose interference has reduced clinical significance. This is in contrast to the case where glucose binding causes hologram swelling when the additional presence of fructose causes a larger than normal shift in the diffraction peak corresponding to a higher glucose concentration than actually present.
The above results highlight the suitability of this approach for physiological glucose determination. Importantly, the sensor does not respond to maltose, which is present in many parenteral products and has been found to interact with numerous commercial electrochemical devices relying on glucose dehydrogenase and pyrroloquinolinequinone (GDH-PQQ). This has led to false readings of hyperglycaemia and unnecessary insulin administration, resulting in injury and death.18 Furthermore, the sensor does not respond to proteins (e.g. albumin, lysozyme, α1-glycoprotein, IgG) due to their large size and inability to penetrate into the crosslinked hydrogel matrix (data not shown). Further studies are in progress to assess the behaviour of holographic glucose sensors in blood and plasma.
Notes and references
- A. G. Mayes, J. Blyth, R. B. Millington and C. R. Lowe, Anal. Chem., 2002, 74(15), 3649 CrossRef CAS.
- A. G. Mayes, J. Blyth, M. Kyrolainen-Reay, R. B. Millington and C. R. Lowe, Anal. Chem., 1999, 71, 3390 CrossRef CAS.
- A. J. Marshall, J. Blyth, C. A. B. Davidson and C. R. Lowe, Anal. Chem., 2003, 75, 4423 CrossRef CAS.
- B. Madrigal Gonzalez, G. Christie, C. A. B. Davidson, J. Blyth and C. R. Lowe, Anal. Chim. Acta, 2005, 528(2), 219 CrossRef.
- A. J. Marshall, D. S. Young, S. Kabilan, A. Hussain, J. Blyth and C. R. Lowe, Anal. Chim. Acta, 2004, 527, 13 CrossRef CAS.
- A. J. Marshall, D. S. Young, J. Blyth, S. Kabilan and C. R. Lowe, Anal. Chem., 2004, 76(5), 1518 CrossRef CAS.
- A. M. Horgan, A. J. Marshall, S. J. Kew, K. E. S. Dean, C. D. Creasey and S. Kabilan, Biosens. Bioelectron., 2006, 21, 1838 CrossRef CAS.
- Diabetes Control and Compilations Trial Research Group, The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes, New Engl. J. Med., 1993, 329, 977 CrossRef.
- C. J. Ward, P. Patel and T. D. James, Org. Lett., 2002, 4(4), 477 CrossRef CAS.
- R. Badugu, J. R. Lakowicz and C. D. Geddes, Analyst, 2004, 129, 516 RSC.
- J. P. Lorand and J. O. Edwards, J. Am. Chem. Soc., 1959, 24, 769 CAS.
- K. R. A. S. Sandanayake, T. D. James and S. Shinkai, Chem. Lett., 1995, 503 CAS.
- V. V. Karnati, X. Gao, S. Gao, W. Yang, W. Ni, S. Sankar and B. Wang, Bioorg. Med. Chem. Lett., 2002, 12, 3373 CrossRef CAS.
- C. Bresner, S. Aldridge, I. A. Faliis, C. Jones and L.-L. Ooi, Angew. Chem., Int. Ed., 2005, 44, 3606 CrossRef CAS.
- L. I. Bosch, M. F. Mahon and T. D. James, Tetrahedron Lett., 2004, 45, 2859 CrossRef CAS.
- T. Kawasaki, H. Akanuma and T. Yamanouchi, Diabetes Care, 2002, 25(2), 353 Search PubMed.
- T. Kawasaki, N. Ogata, H. Akanuma, T. Sakai, H. Watanabe, K. Ichiyanagi and T. Yamanouchi, Metabolism, 2004, 53(5), 583 CrossRef CAS.
- Institute for Safe Medication Practices, Be aware of false glucose results with point-of-care testing, ISMP Medication Safety Alert, 2005, 10, 18: 8 Search PubMed.
Footnote |
| † Thin polymer hydrogel films were prepared on 3-(trimethoxysilyl)propyl methacrylate silanised glass slides by UV polymerisation of 3-acrylamidophenylboronic acid (3-APB), N,N′-methylenebisacrylamide (MBA), N-[3-(dimethylamino)propyl]-acrylamide (DAPA) and acrylamide in dimethyl sulfoxide containing 2,2-dimethoxy-2-phenylacetophenone photoinitiator at the required molar ratios. Holographic gratings were fabricated by diffusing AgNO3, then KBr and 1,1′-diethyl-2,2′-cyanine iodide photosensitizer into the hydrogel films, resulting in insoluble, light sensitive crystals of AgBr. The films were then placed between two mirrors and exposed to collimated laser irradiation. Interference between the incident and reflected beams selectively sensitises AgBr for conversion to metallic silver. Subsequent photographic development and fixing results in the production of silver fringes perpendicular to the incident beam. Acting as a diffraction grating, the silver fringes selectively reflect light of a narrow band of wavelengths. As the interlayer spacing of a holographic grating changes due to the volumetric changes of the hydrogel matrix resulting from receptor–analyte binding, the wavelength of diffracted light changes according to Bragg's law (λmax = 2nd sin θ). The sugars used in this work were: D-(−)-fructose, D-(+)-galactose, D-(+)-maltose, D-(+)-mannose, D-(+)-glucose, D-(−)-ribose, D-(+)-xylose, lactose and sucrose. The holographic response was monitored using a single strip of hologram inserted into a plastic cuvette with the film side facing inward. PBS (10 mM phosphate, 137 mM NaCl, 2.7 mM KCl, pH 7.4) (1 mL) was added and the cuvette left to equilibrate at 30 °C with stirring. A reflection spectrophotometer was used to measure the wavelength of light reflected from a white light source by the back of the hologram. The response of the sensor to the addition of aliquots of 0.1 M sugar in PBS was recorded. Between each addition, the sensor was allowed to equilibrate to a stable diffraction wavelength. |
| This journal is © The Royal Society of Chemistry 2006 |
