Fluorinated liquid crystals formed by halogen bonding†
Pierangelo
Metrangolo
*ab,
Carsten
Präsang
c,
Giuseppe
Resnati
*ab,
Rosalba
Liantonio
ab,
Adrian C.
Whitwood
c and
Duncan W.
Bruce
*c
aNFMLab-DCMIC “G. Natta”, 7 via Mancinelli, I-20131 Milan, Politecnico di Milano, Italy. E-mail: pierangelo.metrangolo@polimi.it; giuseppe.resnati@polimi.it; Tel: +39 02 2399 3041 (PM), 3032 (GR); Fax: +39 02 2399 3080; Web: http://nfmlab.chem.polimi.it
bCampus of Como, 7 via Castelnuovo, I-22100 Como, Politecnico di Milano, Italy
cDepartment of Chemistry, University of York, Heslington, YORK, UK YO10 5DD. E-mail: db519@york.ac.uk; Tel: +44 1904 434085
First published on 18th May 2006
Abstract
New, halogen-bonded fluorinated mesogens are reported; the expected microphase separation associated with perfluoroalkyl chains is surprisingly absent in the mesophase.
Fluorination has for a long time been a productive strategy for exerting control over both mesomorphism and physical properties in liquid crystals.1 In particular, lateral fluorination has been used to exert control over melting points, mesomorphism and the sign of properties such as dielectric anisotropy, a particularly fine example being the work undertaken on 4,4″-disubstituted terphenyl derivatives.1,2 It was also found that liquid crystals can derive their molecular dipole moment from terminal substitution with perfluoroalkyl (Rf) chains. These so-called superfluorinated materials (SFMs) are typically characterised by a strong dielectric anisotropy and high melting temperatures, and also fulfil certain requirements for use in modern liquid crystal displays.3
Generally, the introduction of long Rf chains in mesogens enhances the smectic character of a liquid crystal,4 and the smectic A (SmA) phase can be stabilised even for compounds with a core that consists of a single benzene unit, owing to the stiffness of long Rf chains.5 Thus, a major feature of the mesomorphism of compounds containing long Rf chains is the dominance of lamellar phases and the effective absence of the nematic phase due to the microphase separation that occurs between these chains and other segments of the molecule (fluorophobic effect).4 For this reason, the fluorophobic effect could lead to a significant stabilisation and even to modifications of smectic, columnar, and cubic mesophases.
In recent years the number of reports in the literature on the use of Rf chains has risen consistently, perhaps prompted in part by reports of possible symmetry-breaking in the SmA phase of materials containing Rf chain segments.6 For example, our own work with four-ring imine mesogens containing two, terminal perfluorinated chains revealed a new class of materials showing cubic phases.7,8 More recently, such general ideas on the self-organisation of fluorinated segments have also found expression in the search for materials showing the biaxial nematic phase.9,10
New families of liquid-crystalline materials have been developed by the identification of non-covalent interactions such as hydrogen bonding,11 quadrupolar12 and charge-transfer13 interactions that can lead to new mesomorphic supramolecular species by self-assembly. Most recently, the halogen bond has proven successful in determining the self-assembly of supramolecular mesogens.
The halogen bond14 is a charge-transfer interaction directly analogous to the hydrogen bond, and major contributions to the development of its scope and chemistry have been driven by the Milan group.15 Halogen-bonded interactions require the presence of an electron-poor halogen (normally iodine or bromine) and an electron-rich Lewis base (often an amine or pyridine derivative), and a range of structures has been described.16 Recently, some of us reported17 that halogen bonding could also be used to induce liquid crystal phase behaviour by combination of a non-mesomorphic alkoxystilbazole with iodopentafluorobenzene (Fig. 1), where for n = 4 the complexes showed a monotropic nematic phase, while for n = 12, an enantiotropic SmA phase was seen. Subsequently, supramolecular polymeric liquid crystals based on halogen bonding have been reported.18 Keen to extend this work and having shown examples of dimeric complexes formed by single halogen bonds, we then undertook a low molar mass approach to trimeric systems based on two halogen bonds and investigated the complexation of a range of alkoxystilbazoles to α,ω-diiodoperfluoroalkanes. Reports regarding supramolecular mesogens obtained on combining hydrogen bonding and the fluorophobic effect are very rare in the literature.19 Here we report the first case of liquid crystals formed by combining halogen bonding and the use of iodoperfluoroalkanes.
 | ||
| Fig. 1 Halogen-bonded liquid crystals. | ||
Complexes were obtained by crystallising from THF solution a 2∶1 mixture of the stilbazole and the diiodoperfluoroalkane to give the products 1, shown in Fig. 2, which have been fully characterised (see ESI†). The complexes were pale yellow in colour, indicating a degree of charge-transfer to the stilbazole chromophore, whose absorption has been shown to shift on charge-transfer complexation.20Fig. 3 shows two views of the structure of the complex 1d as determined by single crystal X-ray diffraction methods.‡ The ratio of stilbazole to 1,6-diiodoperfluorohexane in the crystal is 2∶1 as can be anticipated from the telechelic halogen bond donor behaviour of 1,6-diiodoperfluorohexane. The adduct is characterised by a stepped arrangement between the two stilbazoles, a consequence of the antiperiplanar arrangement of the perfluoromethylene groups of the halogen bond donor. There is an inversion centre about the centre of the central C–C bond of the diiodoperfluorohexane, and so there is only one N⋯I distance, found to be 2.805(2) Å, which corresponds to a reduction of about 20% with respect to the sum of van der Waals radii for N and I (3.53 Å),21 with a N⋯I–C angle of 176.71(8)°. Such parameters are typical of those found in the literature of halogen-bonded materials.16 Analysis of the crystal packing of complex 1d shows how the stilbazole and the perfluorinated modules stack in separated columns parallel to the a crystallographic axis (Fig. 4), probably as a consequence of the fluorophobic effect. However, multiple H⋯F intercolumnar interactions (distances between 2.498–2.659 Å) stabilise the crystal packing.
 | ||
| Fig. 2 Structure of the new halogen-bonded complexes 1. | ||
 | ||
| Fig. 3 Two ball-and-stick views of the single crystal X-ray structure of the halogen-bonded trimeric complex 1d. | ||
 | ||
| Fig. 4 Partial view of the crystal packing of the complex 1d showing microphase separation between the Rf chains and the hydrocarbon molecules. The diiodoperfluorohexane and stilbazole modules stack in columns, which run parallel to the a crystallographic axis. | ||
For all of the complexes 1 the ratio between the stilbazole and the diiodoperfluoroalkane used was established by recording their 1H and 19F NMR spectra, respectively, in the presence of 2,2,2-trifluoroethyl ether as an internal standard. On calibrating integration parameters so that in the 1H NMR spectrum the CH2O quartet of 2,2,2-trifluoroethyl ether corresponded to four hydrogens and that in the 19F NMR spectrum the CF3 triplet of 2,2,2-trifluoroethyl ether corresponded to six fluorines, the ratio between the –CH3 signal area (derived from the stilbazole) and the –CF2I signal area (derived from the diiodide) was 3∶2, thus revealing that the stilbazole∶diiodide ratio in all of the complexes 1 is 2∶1 (see ESI†).15
The liquid crystal properties (Table 1) of the new complexes were studied by hot stage polarising optical microscopy and differential scanning calorimetry (DSC). On heating, all of the complexes 1a–f melted directly to the isotropic liquid, yet the process occurred over several degrees and showed apparent biphasic behaviour with the presence of both an isotropic liquid and crystals floating therein. We will return to this point presently, but interestingly, this melting point increased with the chain length of the diiodoperfluoroalkane and decreased with the chain length of the stilbazole used, with the exception of 1e. The former behaviour is apparently in contrast with previous observations of melting points that are always lower for halogen-bonded complexes of 1,6-diiodoperfluorohexane compared to those of 1,4-diiodoperfluorobutane.22 Once an apparently homogeneous isotropic state was reached, the samples were cooled, and for the examples shown in Table 1, a monotropic nematic phase was seen, evident from its characteristic schlieren texture (Fig. 5), with the only exception of 1e. The samples heated were, in each case, single crystal in nature (structures have been obtained for >1 of the materials described in this communication) and so the behaviour on melting can be ascribed to initial breakdown of the halogen-bonded complex followed by its re-formation, probably in the isotropic phase. This is evidenced by the fact that, in the non-mesomorphic materials, crystallisation occurred at temperatures that were well above the clearing points of the related stilbazoles and shows the high thermal stability of the halogen bond. Such behaviour is allowed thermodynamically, simply reflecting the lower Gibbs function for the mixture of crystals and the isotropic phase, than for the single isotropic phase at these temperatures; this behaviour has literature precedent.23 In most cases, the nematic phase was observed over a range of perhaps 3–5 °C before the material crystallised, after which the thermal behaviour could be reversed (i.e. melting to isotropic followed by monotropic N phase). However, for 1c the nematic phase appeared longer lived and reproducibly existed for some 10 °C before crystallisation occurred.
| Complex | Transition | T/°C |
|---|---|---|
| 1a | Cr–I | 102.8 |
| (N–I) | (95.2) | |
| 1b | Cr–I | 99.3 |
| (N–I) | (91.1) | |
| 1c | Cr–I | 95.3 |
| (I–N) | (90.1) | |
| 1d | Cr–I | 108.4 |
| (N–I) | (103.5) | |
| 1e | Cr–I | 110.4 |
| 1f | Cr–I | 99.7 |
| (I–N) | (95.8) |
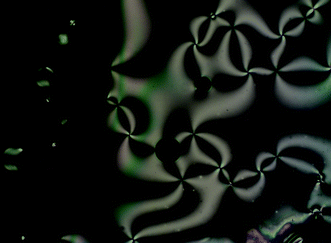 | ||
| Fig. 5 Optical texture of the monotropic nematic phase of 1b at 91 °C. | ||
What is intriguing about these materials is that they all show the nematic phase. The mesomorphism of the halogen-bonded stilbazole complexes reported earlier (Fig. 1)17 was that of a simple, dipolar mesogen, showing a nematic phase at short chain length and a SmA phase with longer chains. Similar behaviour was found for cis-[MCl(CO)2]-functionalised stilbazoles (M = Rh, Ir).24 However, as suggested earlier, the mesomorphism of mesogens containing perfluoroalkyl chains is dominated by the formation of smectic phases – normally SmA1,4 – due to an effective microphase separation between fluorocarbon and other segments of the molecule. Here, this is clearly not the case and even the materials containing a diiodoperfluorohexane spacer show a nematic phase. One possible origin might be the flexibility in the N⋯I link, although we suspect that the size of the stilbazole makes this unlikely and another explanation must be sought in due course.
In conclusion, halogen bonding drives the self-assembly of a range of alkoxystilbazoles with α,ω-diiodoperfluoroalkanes into trimeric complexes. And, despite the non-mesomorphic nature of the starting materials, most of the halogen-bonded complexes show liquid-crystallinity. Thus, we have shown the first case of the use of halogen bonding and iodoperfluoroalkanes in the design of new, supramolecular, fluorinated liquid crystals. The use of shorter and longer chain diiodoperfluoroalkanes than those reported in this communication, as well as the use of mono-iodoperfluoroalkanes are currently under study for the synthesis of new halogen-bonded fluorinated liquid crystals.
The authors thank the Royal Society of Chemistry (‘Journals Grant for International Authors’ to PM), Fondazione Cariplo (RL) and EPSRC (CP) for funding.
Notes and references
- M. Hird and K. J. Toyne, Mol. Cryst. Liq. Cryst., 1998, 323, 1 CrossRef CAS
; F. Guittard, E. Taffin de Givenchy, S. Geribaldi and A. Cambon, J. Fluorine Chem., 1999, 100, 95
.
- See e.g., G. W. Gray, M. Hird and K. J. Toyne, Mol. Cryst. Liq. Cryst., 1991, 204, 43 Search PubMed
.
- P. Kirsch and M. Bremer, Angew. Chem., Int. Ed., 2000, 39, 4216 CrossRef CAS
and references cited therein.
- M. Yano, T. Taketsugu, K. Hori, H. Okamoto and S. Takenaka, Chem.–Eur. J., 2004, 10, 3991 CrossRef CAS
and references cited therein.
- As an example, G. Johansson, V. Percec, G. Ungar and K. Smith, Chem. Mater., 1997, 9, 164 Search PubMed
.
- F. Tournilhac, L. M. Blinov, J. Simon and S. V. Yablonsky, Nature, 1992, 359, 621 CrossRef CAS
.
- M. A. Guillevic and D. W. Bruce, Liq. Cryst., 2000, 27, 153 CrossRef CAS
.
- M. A. Guillevic, T. Gelbrich, M. B. Hursthouse and D. W. Bruce, Mol. Cryst. Liq. Cryst., 2001, 362, 147 CrossRef CAS
.
- L. Omnès, B. A. Timimi, T. Gelbrich, M. B. Hursthouse, G. R. Luckhurst and D. W. Bruce, Chem. Commun., 2001, 2248 RSC
.
- L. Omnès, V. Cîrcu, P. T. Hutchins, S. J. Coles, M. B. Hursthouse and D. W. Bruce, Liq. Cryst., 2005, 32, 1437 CrossRef CAS
.
-
T. Kato, in Handbook of Liquid Crystals, ed. D. Demus, G. W. Gray, J. Goodby, H.-W. Spiess and V. Vill, Wiley-VCH, Weinheim, 1998 Search PubMed
; D. Tsiourvas and C. Paleos, Angew. Chem., Int. Ed. Engl., 1995, 34, 1696 Search PubMed
.
- N. Boden, R. J. Bushby, Z. Lu and O. R. Lozman, Liq. Cryst., 2001, 28, 657 CrossRef CAS
.
- See e.g., K. Praefcke and J. D. Holbrey, J. Inclusion Phenom. Mol. Recognit. Chem., 1996, 24, 19 Search PubMed
.
- A. C. Legon, Angew. Chem., Int. Ed., 1999, 38, 2687 CrossRef CAS
; A. C. Legon, Chem.–Eur. J., 1998, 4, 1980
.
- P. Metrangolo and G. Resnati, Chem.–Eur. J., 2001, 7, 2511 CrossRef
.
- P. Metrangolo, H. Neukirch, T. Pilati and G. Resnati, Acc. Chem. Res., 2005, 38, 386 CrossRef CAS
.
- H. L. Nguyen, P. N. Horton, M. B. Hursthouse, A. C. Legon and D. W. Bruce, J. Am. Chem. Soc., 2004, 126, 16 CrossRef CAS
.
- J. Xu, X. Liu, T. Lin, J. Huang and C. He, Macromolecules, 2005, 38, 3554 CrossRef CAS
.
- See e.g., A. Kohlmeier and D. Janietz, Chem. Mater., 2006, 18, 59 Search PubMed
.
- D. J. Price, T. Richardson and D. W. Bruce, J. Chem. Soc., Chem. Commun., 1995, 1911 RSC
.
-
http://www.webelements.com
.
- A. Lunghi, P. Cardillo, M. T. Messina, P. Metrangolo, W. Panzeri and G. Resnati, J. Fluorine Chem., 1998, 91, 191 CrossRef CAS
; P. Cardillo, E. Corradi, A. Lunghi, S. V. Meille, M. T. Messina, P. Metrangolo and G. Resnati, Tetrahedron, 2000, 56, 5535 CrossRef CAS
.
- D. J. Price, H. Adams and D. W. Bruce, Mol. Cryst. Liq. Cryst., 1996, 289, 127 CrossRef CAS
.
- D. W. Bruce, D. A. Dunmur, M. A. Esteruelas, S. E. Hunt, R. Le Lagadec, P. M. Maitlis, J. R. Marsden, E. Sola and J. M. Stacey, J. Mater. Chem., 1991, 1, 251 RSC
.
-
(a)
G. M. Sheldrick, SHELXL-97, Program for refinement of crystal structures, University of Göttingen, Germany, 1997 Search PubMed
; (b) G. M. Sheldrick, SHELXS-97, Program for solution of crystal structures, University of Göttingen, Germany, 1997 Search PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedure and CIF for 3d. See DOI: 10.1039/b605101d |
‡ Crystal data for1d: C24H27F6INO, M
= 586.37 g mol−1, yellow blocks, 0.36 × 0.20 × 0.10 mm3, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a
= 7.9751(4)
Å, b
=12.3703(6)
Å, c
= 12.7345(6)
Å, α
= 105.832(1)°, β
= 97.487(1)°, γ
= 96.492(1)°, V
= 1183.72(10)
Å3, Z
= 2, Dc
= 1.645 g cm−3, F000 = 586, MoKα radiation, λ
= 0.71073 Å, μ
= 1.417 mm−1, T
= 110(2) K, 2θmax
= 60.02°, 13476 reflections collected, 6656 unique (Rint
= 0.0165), 6211 with I0 > 2σ(I0), absorption corrections Tmin/Tmax
= 0.880. Solved using SHELXS-9725b and refined with SHELXL-9725a with full-matrix least squares on F2, 299 parameters, GoF
= 1.049, R1[I > 2σ(I0)]
= 0.0236, wR2[I > 2σ(I0)]
= 0.0587, R1
(all reflections)
= 0.0259, wR2
(all reflections)
= 0.0598, −0.52 < Δρ < 1.17 eÅ−3. CCDC 604155. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b605101d , a
= 7.9751(4)
Å, b
=12.3703(6)
Å, c
= 12.7345(6)
Å, α
= 105.832(1)°, β
= 97.487(1)°, γ
= 96.492(1)°, V
= 1183.72(10)
Å3, Z
= 2, Dc
= 1.645 g cm−3, F000 = 586, MoKα radiation, λ
= 0.71073 Å, μ
= 1.417 mm−1, T
= 110(2) K, 2θmax
= 60.02°, 13476 reflections collected, 6656 unique (Rint
= 0.0165), 6211 with I0 > 2σ(I0), absorption corrections Tmin/Tmax
= 0.880. Solved using SHELXS-9725b and refined with SHELXL-9725a with full-matrix least squares on F2, 299 parameters, GoF
= 1.049, R1[I > 2σ(I0)]
= 0.0236, wR2[I > 2σ(I0)]
= 0.0587, R1
(all reflections)
= 0.0259, wR2
(all reflections)
= 0.0598, −0.52 < Δρ < 1.17 eÅ−3. CCDC 604155. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b605101d |
| This journal is © The Royal Society of Chemistry 2006 |
