Extractive electrospray ionization for direct analysis of undiluted urine, milk and other complex mixtures without sample preparation
Huanwen
Chen
,
Andre
Venter
and
R. Graham
Cooks
*
560 Oval Drive, Chemistry Department, Purdue University, West Lafayette, Indiana 47907, USA. E-mail: Cooks@purdue.edu; Fax: +1 765 494 9421; Tel: +1 765 494 5263
First published on 10th April 2006
Abstract
On-line droplet–droplet extraction occurs when a sample spray intersects a reagent electrospray; this allows continuous analysis of trace amounts of compounds directly in complex matrices including undiluted urine, milk and polluted water over extended periods of time.
Detection of analytes as diverse as pesticides and explosives in complex matrices is of increasing importance in analytical chemistry, driven by threats to the living environment and to civil society.1 These applications demand rapid, sensitive and selective analytical techniques. Reducing or removing sample preparation steps prior to analysis is key to moving the analysis of complex samples out of the lab and towards automated, in situ protocols. Some of the most promising techniques for direct, real time analysis are based on new mass spectrometry methods in which samples are ionized in the ambient environment. Additional specificity is then possible from tandem mass spectrometry, high resolution, or ion mobility measurements.2 Ambient ionization methods are already emerging on fieldable instruments.3
A recently developed MS ionization technique, desorption electrospray ionization (DESI)4 allows the examination of compounds directly from ambient surfaces, eliminating solvent extraction or other sample preparation steps prior to analysis. DESI is one of a family of ambient ionization methods which share the advantage that ion production occurs in air where the sample is fully accessible during analysis.
Components of urine and other complex matrices can be analyzed successfully by DESI as dried spots on paper or other surfaces.5 This approach allows whole urine to be examined, eliminating clean-up steps e.g. removal of salts that restrict the application of other ionization methods to this important sample type. While examination of dried spots on paper is a feasible approach to high throughput analysis of fluids, it may not be useful for fragile compounds or in circumstances in which real time measurements are required. Electrospraying samples such as urine, serum, polluted water and milk directly into the inlet of a mass spectrometer causes adduct formation, sample carry-over and build-up of nonvolatile components and quickly leads to irrecoverable loss in sensitivity. This problem has been addressed by directing the spray off-axis with respect to the MS inlet or sampling cone but many samples still need to be worked-up or diluted before the mass spectrometer can be used for extended periods in the analysis of complex biological samples. Such steps are unsatisfactory to many investigators.
In this communication we introduce a new electrospray-based ionization technique that permits the direct analysis of trace compounds in complex matrices such as raw urine, milk and polluted water for long periods of time.† Extractive electrospray ionization (EESI) uses two separate sprayers, one to nebulize the sample solution and the other to produce charged microdroplets of solvent. This approach depends on liquid–liquid extraction between the colliding microdroplets. The compounds of interest are extracted from the analyte solution into the solvent spray in a continuous automatic fashion with no sample preparation steps.
To test the stability of the signal for quantitative analysis, undiluted sample solutions were delivered directly from an infusion pump at flow rates between 1 and 10 µL min−1. The ionizing solvent spray, delivered at 5 and 10 µL min−1, was a mixture of methanol/water/acetic acid (45 ∶ 45 ∶ 10). Both sprayers operate in a mode similar to that used in electrosonic spray ionization (ESSI)6 with dry nitrogen at 200 psi being used as the nebulizing gas. The sprayers were positioned at an angle to the Thermo LTQ mass analyzer inlet and with respect to each other in such a way that the ionized analyte molecules were directed towards the MS inlet by the combined aerodynamic effect of both sprayers. The charged ionizing spray turbulently mixes with the nebulized uncharged sample spray. A typical source geometry is shown in Fig. 1.
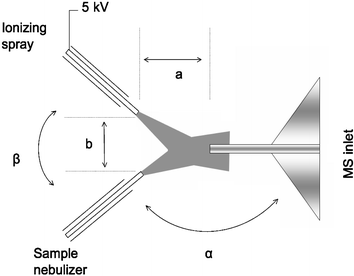 | ||
| Fig. 1 Schematic diagram of Extractive Electrospray Ionization showing ionizing and sample sprays. | ||
Acceptable results were obtained for many combinations of the angle between the sample nebulizer and MS inlet (α) and the angle between the two sprayers (β). Generally there is a trade-off between high sensitivity, which is favored by large values for α, and long term stability favored by values of α approaching 90°. The urine analysis data in Fig. 2 were taken at α = β = 90° whereas the low detection level data reported in Figs. 3 and 4 were obtained with α = 120° and β = 60°. The optimal distance between the sprayers (a) varies as a function of spray angle while a typical value for the sprayers to inlet distance (b) is 20 mm. The solvent sprayer was connected to a high voltage power supply and spray voltages of 3 to 5 kV were used in both the positive and negative ion modes. The analytes are ionized without compromising the analytical performance of the mass spectrometer, even after prolonged exposure to the complex matrix.
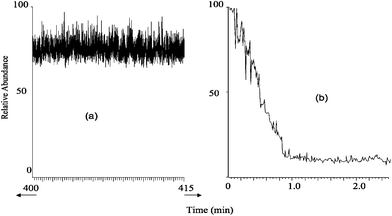 | ||
| Fig. 2 (a) Long term stability of raw urine signal spiked with 2 × 10−9 mol·L−1 atrazine; showing a 15 min region towards the end of a 7 hour EESI run. (b) A similar analysis with APCI of a diluted urine sample (1 ∶ 1000) with a micro APCI source. | ||
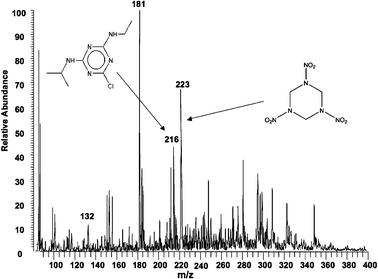 | ||
| Fig. 3 Extractive electrospray ionization of undiluted mouse urine spiked with 1 × 10−9 M atrazine and 1 × 10−12 M RDX. (Average of four 200 ms scans). | ||
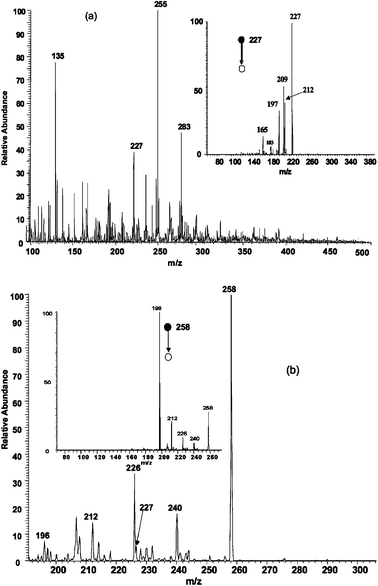 | ||
| Fig. 4 Analysis of 1 × 10−12 M TNT in polluted river water. a) Direct analysis by EESI. The insert shows the MS2 spectrum of m/z 227, the radical anion of TNT. b) Ion/molecule reactions in EESI yield the Meisenheimer complex of TNT (insert shows fragmentation of the Meisenheimer complex). | ||
Electrospray ionization sources in which ionization is spatially separated from sample nebulization have previously been used to ionize small molecules at the exit of a gas chromatograph7 and for proteins in pure water solutions by use of a piezoelectric nebulizer.8,9 In these experiments, free gas-phase molecules were made to interact with electrosprayed droplets in a glass reaction vessel before sampling by the mass spectrometer. In a recent report, a fused droplet electrospray source was used to remove interferences from sodium dodecyl sulfate (SDS) and tris(hydroxymethyl)aminomethane buffer (TRIS) based on the relative solubilities of TRIS, SDS-ion pairs and the protein analytes in methanol.10 Dual- and multi-electrospray sources have also been used for ionization of multiple sample streams to increase throughput,11 to ease the coupling of wide bore HPLC columns with ESI12 sources, to increase sensitivity,13 for ion/ion reactions14 and to add mass calibrants for accurate mass analyses.15
The present work applies the concept of multiple sprayers and two-stage ionization in a novel fashion and to our knowledge represents the first example of mass spectrometric analysis based on transfer of analyte from solution to a solvent. Most importantly, it allows prolonged continual analysis of complex biological and environmental matrices without any sample preparation.
Fig. 2 demonstrates the signal stability of EESI over long times by showing part of the total ion chronogram (TIC) obtained for raw, undiluted human urine analyzed for seven consecutive hours. Also shown is the TIC for a 1 ∶ 1000 dilute sample of urine infused at 1 µL min−1 and ionized using a microspray APCI source.17 For the EESI analysis the sample was infused at 5 µL min−1 with a 250 µL glass syringe. Sharp negative spikes occurred at 70, 130, 190, 250, 320 and 390 minutes due to artifacts caused when the syringe was refilled. While the signal appears noisy as compressed here, it is stable over the few seconds required for individual sample analyses. Mass spectra recorded at any time show numerous compounds present in raw urine necessitating the use of tandem MS analysis for identification and quantification of target analytes. These mass spectra did not change appreciably from the beginning to the end of the 7-hour experiment. Other sample types successfully analyzed include undiluted milk and serum. The stability and signal intensity depend on the relative positioning of the sprayers.
Apart from long term stability, extractive electrospray ionization is also very sensitive. The direct monitoring of atrazine at low levels is demonstrated in Fig. 3. The product ion spectrum of protonated atrazine (m/z 216) yields a main fragment ion of m/z 174 after loss of CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2. This is followed by the loss of neutral CH2
CH2. This is followed by the loss of neutral CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 to produce the m/z 146 fragment ion. Very low levels of RDX and atrazine were also observed by extractive electrospray ionization in undiluted mouse urine as shown in Fig. 3. Many of the components typically found in mammalian urine were observed, for example creatine (m/z 132) and glucose (m/z 181).
CH2 to produce the m/z 146 fragment ion. Very low levels of RDX and atrazine were also observed by extractive electrospray ionization in undiluted mouse urine as shown in Fig. 3. Many of the components typically found in mammalian urine were observed, for example creatine (m/z 132) and glucose (m/z 181).
2,4,6-Trinitrotoluene (TNT) was readily detected in the negative ion mode using EESI mass spectrometry in a polluted river water sample spiked with TNT at a concentration of 1 × 10−12 M. The radical anion corresponding to TNT (m/z 227) occurs in the mass spectrum and was confirmed by CID (Fig. 4a).
Ion/molecule reactions can be deliberately performed during the droplet collision event at atmospheric pressure. These reactions can be used to improve detection levels or to confirm the presence of analytes in complex mixtures by selective reactions. Fig. 4b demonstrates the formation of the diagnostic Meisenheimer complex of TNT.16 A solution of 1 ppm sodium methoxide in methanol was used as the ionizing spray to produce CH3O− anions. These reagent ions reacted with TNT in the sample spray to form the Meisenheimer complex at m/z 258. The identity of the ion was confirmed by CID which produced fragments at m/z 240, 226, 212 and 198 due to the loss of water, methanol and ˙CH2NO2, respectively.
It is not known in detail how analytes are ionized in the microspray extraction method or why the signal stability is maintained during the analysis of complex salt-rich biological samples. A simple charge transfer mechanism, from the charged droplets to the desolvated analyte molecules, does not explain the increase in stability, nor does the limited dilution that is obtained by the action of the additional sprayer. A working hypothesis is that non-polar compounds compete for the surface of neutral sample droplets while salts and polar molecules are stabilized by increased solvation at the droplet center. The molecules at the surface of the neutral droplets would then be extracted into the charged droplets during droplet collision events. Systematic study of solvent effects on signal intensity and stability as well as physical measurements by phase doppler anemometry (PDA) and particle image velicometry (PIV) are currently underway to further elucidate the mechanism.
The advantage of extractive electrospray ionization (EESI) is evident by the rapid loss in signal intensity observed in conventional ESI/APCI ion sources when even diluted urine is infused. By contrast, the dual spray methodology offers good tolerance even to undiluted urine samples flowing at similar rates for very long periods. No significant loss in signal was observed after many hours of analysis of raw urine. Under optimal conditions, the microspray extraction procedure provides sensitivity approaching or exceeding that of ESI-MS but with the continuous operation already noted. The inherent flexibility of the double sprayer configuration offers the ability to perform ion/molecule reactions at atmospheric pressure to improve sensitivity and/or selectivity. These features ensure that this new solution ionization method will find application in the analysis of trace compounds present in other complex matrices such as serum. Applications in metabolite profiling for differential metabolomics in biofluids and in manipulation of biopolymer charge states are in progress.
This work was funded by the National Science Foundation (0412782) and the US Army Corps of Engineers.
Notes and references
- F. Hernandez, J. V. Sancho and O. Pozo, J. Anal. Bioanal. Chem., 2005, 382, 934–946 Search PubMed.
- R. G. Ewing, D. A. Atkinson, G. A. Eiceman and G. J. Ewing, Talanta, 2001, 54, 515–529 CrossRef CAS.
- B. C. Laughlin, C. C. Mulligan and R. G. Cooks, Anal. Chem., 2005, 77, 2928–2939 CrossRef CAS.
- Z. Takáts, J. M. Wiseman and R. G. Cooks, J. Mass Spectrom., 2005, 40, 1261–1275 CrossRef CAS.
- H. W. Chen, N. N. Talaty, Z. Takats and R. G. Cooks, Anal. Chem., 2005, 77, 6915–6927 CrossRef CAS.
- Z. Takats, J. M. Wiseman, B. Gologan and R. G. Cooks, Anal. Chem., 2004, 76, 4050–4058 CrossRef CAS.
- C. Lee and J. Shiea, Anal. Chem., 1998, 70, 2757–2761 CrossRef CAS.
- C. Lee, D. Chang, J. Jeng and J. Shiea, J. Mass Spectrom., 2002, 37, 115–117 CrossRef CAS.
- J. Shiea, D. Chang, C. Lin and S. Jiang, J. Anal. Chem., 2001, 73, 4983–4987 CAS.
- I. Shieh, C. Lee and J. Shiea, J. Protein Res., 2005, 4, 606–612 Search PubMed.
- B. B. Schneider, D. J. Douglas and D. Y. Chen, Rapid Commun. Mass Spectrom., 2002, 16, 1982–1990 CrossRef CAS.
- R. Kostiainen and A. P. Bruins, Rapid Commun. Mass Spectrom., 1994, 8, 549 CAS.
- K. Tang, Y. Lin, D. W. Matson, T. Kim and R. D. Smith, Anal. Chem., 2001, 73, 1658–1663 CrossRef CAS.
- Y. Xia, X. Liang and S. A. McLuckey, J. Am. Soc. Mass Spectrom., 2005, 16, 1750–1756 CrossRef CAS.
- L. Jiang and M. Moini, Anal. Chem., 2000, 72, 20–24 CrossRef CAS.
- H. Chen, H. W. Chen and R. G. Cooks, J. Am. Soc. Mass Spectrom., 2004, 15, 998–1004 CrossRef CAS.
- H. Chen, Z. Pan, N. Talaty, S. Zhang, C. Duda, R. G. Cooks and D. Raftery, Rapid Commun. Mass Spectrom., 2006 Search PubMed , in press.
Footnote |
| † Biohazard: appropriate precautions should be taken when spraying solutions of biological materials. |
| This journal is © The Royal Society of Chemistry 2006 |
