Controlled release of volatile aldehydes and ketones by reversible hydrazone formation – “classical” profragrances are getting dynamic
Barbara
Levrand
a,
Yves
Ruff
b,
Jean-Marie
Lehn
*b and
Andreas
Herrmann
*a
aFirmenich SA, Division Recherche et Développement, B.P. 239, CH-1211 Genève 8, Switzerland. E-mail: andreas.herrmann@firmenich.com
bISIS, Université Louis Pasteur, 8 allée Gaspard Monge, B.P. 70028, F-67083 Strasbourg cedex, France. E-mail: lehn@isis.u-strasbg.fr
First published on 3rd April 2006
Abstract
Dynamic mixtures obtained by reversible covalent acylhydrazone formation of fragrance aldehydes and/or ketones and a hydrazide in water were found to be efficient delivery systems for the controlled release of highly volatile organic molecules.
For optimal performance, biologically active substances such as pharmaceuticals, agrochemicals, and also flavours or fragrances have to be efficiently delivered to their target site and usually released at a well-defined rate. In the case of fragrances the target sites are different types of surfaces from which the active compound then evaporates. Fragrances are very volatile and their perception is therefore limited in time. Furthermore, many of them, in particular aldehydes, are unstable and undergo partial degradation prior to their effective use. To circumvent these problems, fragrance precursors (so called “profragrances”) have been developed which are cleaved under mild reaction conditions during application.1,2 These precursors thus have to be reasonably stable during product storage but relatively labile once deposited on the target surface such as skin, hair or fabric, in order to release the active compound.
The successful use of reversible covalent reactions in the development of dynamic combinatorial libraries,3–5 in particular for drug discovery,5 prompted us to adapt this concept to the controlled delivery of fragrances. For our investigations we chose the reaction of hydrazine derivatives (namely hydrazides) with aldehydes or ketones which are in an equilibrium with the corresponding hydrazones via an intermediate hemiaminal in a two-step mechanism (Scheme 1).6–8 This reaction is pH dependent and, at neutral pH, the dehydration of the hemiaminal was found to be the rate-determining step, whereas at lower pH hemiaminal formation is the slower step.6–8
 | ||
| Scheme 1 Equilibrium for the reversible hydrazone formation. | ||
To screen the potential of reversible hydrazone formation for controlled fragrance delivery, we used a series of commercially available hydrazides such as benzhydrazide (1), furoic acid hydrazide (2) or the Girard-T reagent9 (3) (Scheme 2), and prepared their corresponding acylhydrazones 4–6 by heating them with a slight excess of benzaldehyde, vanillin or trans-cinnamaldehyde in ethanol, respectively.10 The products crystallised on cooling to room temperature, and could thus be easily isolated.11 The choice of the aldehyde was essentially influenced by the ease of measurement of the kinetic rate constants by UV/Vis spectroscopy at different pH, so as to allow the analysis of the reaction mixture at equilibrium.
 | ||
| Scheme 2 Structures of acylhydrazones 4–6 obtained by reaction of hydrazides 1–3 with different perfumery aldehydes. | ||
Kinetic measurements were carried out in buffered solutions of water/ethanol 2 ∶ 1 at pH 2.47 (phosphate buffer) and 4.48 (citrate buffer), and at a product concentration of ca. 1.7 × 10−5 M. UV/Vis spectra were recorded at constant time intervals between 240 and 450 nm. All reactions were investigated at equimolar concentrations for both formation and hydrolysis of the acylhydrazones. Good linear fits of the data points (r2 > 0.99) were generally obtained by plotting the logarithm of the difference of absorption at time t + Δt and time t (At + Δt − At) against time (Guggenheim method),12 thus indicating first order kinetics.† Within the experimental error, the same equilibrium states were reached either from a mixture of hydrazide and aldehyde or, alternatively, by hydrolysis of the corresponding acylhydrazone as illustrated in Fig. 1 for the reaction between 2, cinnamaldehyde and 5c at pH 2.47.
 | ||
| Fig. 1 Equilibrium reached for the reaction of equimolar amounts of hydrazide 2 with cinnamaldehyde (decrease in absorption at 270 nm and increase at 320 nm) and for the hydrolysis of the corresponding acylhydrazone 5c (increase at 270 nm and decrease at 320 nm) at pH 2.47. | ||
The data in Table 1 show that at pH 2.47 the rates of formation and hydrolysis for a given acylhydrazone are very similar, whereas at pH 4.48 the acylhydrazone formation is generally slightly faster than its hydrolysis. At pH 2.47 the reactions with cinnamaldehyde were found to be slower than those with benzaldehyde or vanillin. This effect was less pronounced at higher pH, and the equilibrations with benzaldehyde occurred slightly faster than those with the other two aldehydes at pH 4.48. As expected, the pH has a much stronger effect on the reaction rates than the structure of the aldehydes or hydrazides. The rate constants for acylhydrazone formation or hydrolysis increased by a factor of 10 to 60 when the pH was decreased by 2 units. This should make this delivery system especially interesting for practical applications which involve a change in pH.
| Formation of acylhydrazone | pH 2.47 | pH 4.48 | Hydrolysis of acylhydrazone | pH 2.47 | pH 4.48 | ||||
|---|---|---|---|---|---|---|---|---|---|
| k obs × 104 [s−1] | t 1/2 [h] | k obs × 104 [s−1] | t 1/2 [h] | k obs × 104 [s−1] | t 1/2 [h] | k obs × 104 [s−1] | t 1/2 [h] | ||
| a ±0.61, r2 > 0.98. b ±0.16. c Observation of a baseline drift in the UV/Vis spectrum, r2 > 0.99. d Average r2 > 0.96. | |||||||||
| 4a | 9.93 | 0.19 | 0.41 | 4.7 | 4a | 10.82 | 0.18 | 0.30 | 6.4 |
| 4b | 8.35a | 0.23 | 0.24 | 8.0 | 4b | 10.79 | 0.18 | 0.18 | 10.7 |
| 4c | 3.34 | 0.58 | 0.31 | 6.2 | 4c | 3.29 | 0.59 | 0.14 | 13.8 |
| 5a | 6.10 | 0.32 | 0.29 | 6.6 | 5a | 6.43 | 0.30 | 0.18 | 10.7 |
| 5b | 6.44b | 0.30 | 0.13 | 14.8 | 5b | 6.61 | 0.29 | 0.11 | 17.5 |
| 5c | 2.57 | 0.75 | 0.23 | 8.4 | 5c | 2.01 | 0.96 | 0.08 | 24.1 |
| 6a | 5.53c | 0.35 | 0.15 | 12.8 | 6a | 5.98 | 0.32 | 0.11 | 17.5 |
| 6b | 5.54 | 0.35 | 0.11d | 17.5 | 6b | 4.94 | 0.39 | 0.09 | 21.4 |
We then investigated the kinetics of evaporation of different fragrance aldehydes and ketones in a dynamic mixture with an acylhydrazone from a surface by dynamic headspace analysis13 under more realistic application conditions. Cationic surfactants, such as quaternised triethanolamine esters of fatty acids (TEA-esterquats), are known to be efficiently deposited onto cotton in aqueous media and thus find use as fabric softening agents.14 With its quaternary ammonium function, hydrazide 3 seemed to be a promising candidate to facilitate the deposition of the dynamic mixture onto the target surface, and was therefore selected to initiate the study of these fragrance delivery systems.
In a simple experiment, a mixture of six fragrance aldehydes and ketones (4-phenyl-2-butanone, (R)-3,7-dimethyl-6-octenal ((+)-citronellal), (±)-3-phenylbutanal, acetophenone, (Z)-4-dodecenal and 10-undecenal) and an equimolar amount of 3 were added to an emulsion of a TEA-esterquat in water (fabric softener base, pH ca. 3.1), and left equilibrating for 5 days to form the dynamic mixture. The sample was then diluted with water (rinsing cycle), which results in an increase of pH of about 1 unit (pH ca. 4.0–4.2) and which is expected to slow down the re-equilibration. To deposit the surfactant together with the hydrazide and the fragrances on the fabric surface, a small cotton sheet was added which was agitated manually for 5 min, then wrung out and air-dried overnight. The fragrance concentration in the headspace above the sample was determined at constant time intervals and compared to a reference sample without hydrazide 3, which was prepared and analysed under the same conditions.‡
The data in Fig. 2 show that the presence of 3 has a significant effect on the evaporation of the fragrance aldehydes and ketones of the mixture. At the end of the experiment (after 7.5 h) the presence of 3 increased the amount of (Z)-4-dodecenal measured in the headspace by a factor of about 1.4, and that of 10-undecenal, 4-phenyl-2-butanone, (±)-3-phenylbutanal and (+)-citronellal by a factor of 4.6, 5.6, 7.6 and 8.8, respectively. In the case of acetophenone the headspace concentration was even found to be 20.5 times higher than for the reference sample without 3. In terms of olfactory features this means that the time release spectrum, and therefore the olfactory perception, are significantly different for the mixtures of the parent fragrance compounds and for the mixture of the dynamic release derivatives. In general, fragrances with vapour pressures above ca. 2 Pa were found to give rise to much higher headspace concentrations than less volatile compounds.15
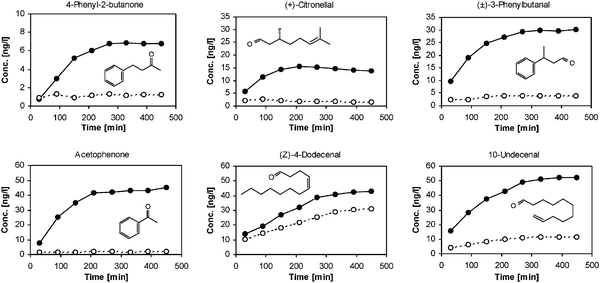 | ||
| Fig. 2 Comparison of the headspace concentrations (in ng/l of air) measured for the evaporation of a mixture of fragrance aldehydes and ketones on a dry cotton sheet in the presence (—●—) or absence (—○—) of hydrazide 3. | ||
The present results show that dynamic mixtures prepared in situ by mixing (perfumery) aldehydes and/or ketones with a hydrazine derivative in water are efficient delivery systems for the controlled release of biologically active compounds. Acylhydrazones are particularly attractive as this functional group incorporates both a peptide bond and a reversible imine unit.16 The dynamic mixtures can easily be deposited on various substrates. Once exposed to a surface in contact with air, the fragrances are removed from the mixture by evaporation, and consequently shift the equilibrium towards the free hydrazine derivative. The gradual hydrolysis of the hydrazones results in a long-lasting fragrance release whose effect is particularly strong for highly volatile odorants. The concept of reversible covalent hydrazone formation to modulate the evaporation of volatile organic molecules from various substrates may find general use in other areas such as the pharmaceutical or agrochemical industry.
We thank Maude Gaillard for assistance in the preparation of some of the compounds, Dr Jean-Yves de Saint-Laumer for the calculation of vapour pressures and Dr Roger Snowden for constructive comments on the manuscript.
Notes and references
- A. Herrmann, The Spectrum (Bowling Green), 2004, 17(2), 10 Search PubMed.
- See for example: H. Kamogawa, H. Mukai, Y. Nakajima and M. Nanasawa, J. Polym. Sci., Polym. Chem. Ed., 1982, 20, 3121 Search PubMed; S. Rochat, C. Minardi, J.-Y. de Saint Laumer and A. Herrmann, Helv. Chim. Acta, 2000, 83, 1645 CrossRef CAS; B. Levrand and A. Herrmann, Photochem. Photobiol. Sci., 2002, 1, 907 CrossRef CAS; J.-Y. de Saint Laumer, E. Frérot and A. Herrmann, Helv. Chim. Acta, 2003, 86, 2871 RSC; J. Lage Robles and C. G. Bochet, Org. Lett., 2005, 7, 3545 CrossRef; C. Fehr and J. Galindo, Helv. Chim. Acta, 2005, 88, 3128 CrossRef.
- For some reviews see: J.-M. Lehn, Chem.–Eur. J., 1999, 5, 2455 Search PubMed; G. R. L. Cousins, S.-A. Poulsen and J. K. M. Sanders, Curr. Opin. Chem. Biol., 2000, 4, 270 CrossRef CAS; S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddard, Angew. Chem., Int. Ed., 2002, 41, 898 CrossRef CAS; S. Otto, R. L. E. Furlan and J. K. M. Sanders, Curr. Opin. Chem. Biol., 2002, 6, 321 CrossRef; S. Otto, Curr. Opin. Drug Discovery Dev., 2003, 6, 509 CrossRef CAS; K. Severin, Chem.–Eur. J., 2004, 10, 2565 Search PubMed; J. D. Cheeseman, A. D. Corbett, J. L. Gleason and R. J. Kazlauska, Chem.–Eur. J., 2005, 11, 1708 CrossRef CAS.
- For recent work see: B. Shi and M. F. Greaney, Chem. Commun., 2005, 886 Search PubMed; T. Hotchkiss, H. B. Kramer, K. J. Doores, D. P. Gamblin, N. J. Oldham and B. G. Davis, Chem. Commun., 2005, 4264 RSC; R. Larsson and O. Ramström, Eur. J. Org. Chem., 2006, 285 RSC.
- J.-M. Lehn and A. V. Eliseev, Science, 2001, 291, 2331 CrossRef CAS; O. Ramström and J.-M. Lehn, Nat. Rev. Drug Discovery, 2002, 1, 26 CrossRef CAS; O. Ramström, T. Bunyapaiboonsri, S. Lohmann and J.-M. Lehn, Biochim. Biophys. Acta, 2002, 1572, 178 CrossRef CAS.
- W. P. Jencks, Prog. Phys. Org. Chem., 1964, 2, 63 CAS; J. M. Sayer, M. Peskin and W. P. Jencks, J. Am. Chem. Soc., 1973, 95, 4277 CrossRef.
- M. Masui and H. Ohmori, J. Chem. Soc. B, 1967, 762 RSC; A. S. Stachissini and L. do Amaral, J. Org. Chem., 1991, 56, 1419 CrossRef CAS; O. H. Wheeler, V. S. Gaind and O. Rosado, J. Org. Chem., 1961, 26, 3537 CrossRef CAS.
- R. Nguyen and I. Huc, Chem. Commun., 2003, 942 RSC.
- A. Girard and G. Sandulesco, Helv. Chim. Acta, 1936, 19, 1095 CrossRef CAS; O. H. Wheeler, Chem. Rev., 1962, 62, 205 CrossRef CAS.
- E. Lederer and G. Nachmias, Bull. Soc. Chim. Fr., 1949, 16, 400; J. R. Dimmock, G. B. Baker and W. G. Taylor, Can. J. Pharm. Sci., 1972, 7, 100 Search PubMed; S. Rao and K. H. Reddy, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 1996, 35, 681; S. B. Said, A. A. Elagamey and R. E. Khadr, Egypt. J. Chem., 2003, 46, 881 Search PubMed.
- Hydrazones 4–6 were obtained with an E configuration at the imine double bond, and as syn isomers (4 and 5) or as a mixture of anti and syn isomers (ca. 1.5–2 ∶ 1, 6) with respect to the amide bond conformation, see: G. Palla, G. Predieri, P. Domiano, C. Vignali and W. Turner, Tetrahedron, 1986, 42, 3649 Search PubMed.
- E. A. Guggenheim, Philos. Mag., 1926, 2, 538 CAS.
- B. Kolb, J. Chromatogr., A, 1999, 842, 163 CrossRef CAS.
- M. I. Levinson, J. Surfactants Deterg., 1999, 2, 223 Search PubMed.
- For the compounds used in this work the following vapour pressures were calculated with the EPIwin v 3.10 program (US Environmental Protection Agency, 2000): 2.4 Pa ((Z)-4-dodecenal), 8.7 Pa (4-phenyl-2-butanone), 8.7 Pa (10-undecenal), 11.4 Pa ((±)-3-phenylbutanal), 37.3 Pa ((+)-citronellal) and 43.5 Pa (acetophenone).
- W. G. Skene and J.-M. P. Lehn, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 8270 CrossRef CAS; J.-M. Lehn, Prog. Polym. Sci., 2005, 30, 814 CrossRef CAS.
Footnotes |
| † Procedure for the kinetic measurements: phosphate and citrate buffer stock solutions (0.15 M, I = 0.1) were prepared in water/ethanol 4 ∶ 1. All UV/Vis measurements were carried out in quartz cuvettes (1 cm) by adding 0.4 ml of hydrazine derivative and aldehyde or the corresponding hydrazone to 2 ml of the buffer stock solution. UV/Vis spectra were recorded at constant time intervals between 240 and 450 nm (every 5 or 10 min at pH 2.47, and every 30 or 60 min at pH 4.48, respectively). The rate constants were determined from the change of absorption measured at 290 nm (benzaldehyde) or 320 nm (cinnamaldehyde and vanillin), with Δt = 1 or 2 h (pH 2.47) or Δt = 7.5 or 15 h (pH 4.48). |
| ‡ Procedure for the headspace analysis: to 1.80 g of a TEA-esterquat (Stepantex®, 16.5% by weight in water) were added 1 ml of ethanol containing the six fragrance molecules (each at 0.041 M), and 1 ml of 3 (0.248 M) in water (or 1 ml of pure water, reference). The sample was closed and left equilibrating for 5 d. Then it was dispersed in a beaker with 600 ml of tap water, and a cotton sheet (ca. 12 × 12 cm) was added. The sheet was agitated manually for 3 min, left standing for 2 min, and wrung out by hand. It was then left drying overnight, put into a home-made headspace sampling cell (160 ml) and exposed to a constant air flow of ca. 200 ml/min at 25 °C. The air was filtered through active charcoal and aspirated through a saturated solution of NaCl. During 15 min the system was left equilibrating, then the volatiles were adsorbed during 15 min onto a clean Tenax® cartridge. The sampling was repeated every hour (8 times). The cartridges were desorbed thermally and analysed by GC-FID. Headspace concentrations (in ng/l of air) were obtained by external standard calibration. |
| This journal is © The Royal Society of Chemistry 2006 |
