Fullerene (C60) immunoconjugates: interaction of water-soluble C60 derivatives with the murine anti-gp240 melanoma antibody†
Jared M.
Ashcroft
a,
Dmitri A.
Tsyboulski
a,
Keith B.
Hartman
a,
Tatiana Y.
Zakharian
a,
John W.
Marks
b,
R. Bruce
Weisman
a,
Michael G.
Rosenblum
b and
Lon J.
Wilson
*a
aDepartment of Chemistry, Smalley Institute for Nanoscale Science and Technology, and the Center for Biological and Environmental Nanotechnology, MS 60, Rice University, Houston, Texas 77005, USA. E-mail: durango@rice.edu; Fax: +1 713-348-5155; Tel: +1 713-348-3268
bImmunopharmacology and Targeted Therapy Section, Department of Experimental Therapeutics, MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, Texas 77030, USA
First published on 9th June 2006
Abstract
The first fullerene (C60) immunoconjugates have been prepared and characterized as an initial step toward the development of fullerene immunotherapy (FIT).
The field of biomedicine offers a promising arena for new applications of fullerene materials.1 Water-soluble C60 derivatives are now commonplace,2 and the discovery that water-soluble C60 derivatives can cross cell membranes3 and even produce transfection4 has accelerated interest in using C60 for diagnostic and therapeutic medicine. Although fullerene toxicity is of some concern, several water-soluble C60 derivatives have shown acceptable cytotoxicity for drug-delivery applications.5
A number of water-soluble C60 derivatives have been suggested for various medical applications. These applications include neuroprotective agents,6 HIV-1 protease inhibitors,7 bone-disorder drugs,8 transfection vectors,4 X-ray contrast agents,9 photodynamic therapy (PDT) agents,10 and a C60–paclitaxel chemotherapeutic.11 In addition, endohedral metallofullerenes have demonstrated potential as radiopharmaceuticals12 and MRI contrast agents.13 Fullerene-based micelles have also been developed as a drug delivery system.14 To date, however, only the bone-drug application has involved biological targeting of a C60-based material,8 even though drug targeting is a desirable, if not essential, component of all drug-delivery strategies.
There is now a large body of literature regarding the development of cell-targeted delivery of agents for imaging and therapeutic applications.15 Growth factors, cytokines and antibodies have all been extensively studied for their abilities to deliver payloads to the surface and the cytoplasm of target cells. The antibody designated ZME-018 targets the gp240 antigen (also known as the high molecular weight melanoma-associated antigen, HMWMAA) found on the surface of >80% of human melanoma cell lines and biopsy specimens.16 This antibody has previously been extensively used in clinical imaging trials17 and for the delivery of toxins, cytokines and other therapeutic agents to melanoma cells in vitro and in vivo.18 Immunoconjugates containing ZME-018 are rapidly internalized into melanoma cells in culture.19 Moreover, these conjugates effectively localize into melanoma xenografts after systemic administration and demonstrate impressive cytotoxic effects against established tumors in orthotopic models.20
In this communication, we report the synthesis and characterization of a new water-soluble C60 derivative (Fig. 1a) designed to covalently attach to proteins such as ZME-018 as an initial step toward targeted fullerene immunotherapy (FIT). A single-drug chemotherapeutic agent such as a recently reported C60–paclitaxel conjugate11 might be employed for FIT, but the real advantage of FIT over other targeted therapeutic agents is the potential for the attachment of multiple (and possibly different) drugs to the C60 scaffold in order to create targeted, single-dose “drug cocktails”.
 | ||
| Fig. 1 The water-soluble fullerene (C60) derivatives. | ||
Several reports have been published regarding C60 interactions with large biomolecules.21 C60 derivatives have been developed to bind myoglobin,21a form electrostatic interactions with cytochrome c,21b,c induce protein clusters and complexes in human serum albumin,21d,e and enhance catalytic activity via conjugation with the serine protease, subtilisin.21f Finally, one study has reported the X-ray crystal structure of a C60-specific monoclonal antibody.21g Together, these studies suggested to us the possibility of creating a C60–antibody conjugate as a proof-of-principle step towards FIT.
Fluorescence spectroscopy and transient absorption spectroscopy have previously been used to detect dendritic C60 interactions with cytochrome c.21c These spectroscopic probes have the advantage of monitoring C60 without interference from the biomolecule. In particular, triplet → triplet (T–T) absorption provides a method to sensitively and selectively monitor C60 derivatives through their known spectral and kinetic signatures.22 We therefore use transient and ground state absorption measurements to track the fullerene components in synthesized immunoconjugates.
The two C60 derivatives shown in Fig. 1 were used in this study. A monoadduct of C60–SPDP (without the water-solubilizing malonodiserinolamide groups of Fig. 1a) was first prepared (see ESI†) to test the feasibility of attaching the cross-linker, N-succinimidyl-3-(2-pyridyldithio)propionate (or SPDP),23 to C60. Water-solubilizing malonodiserinolamide groups were then first attached to C60, followed by the SPDP moiety in Fig. 1a to provide a cross-linking agent for the ZME-018 antibody. A water-soluble derivative of C60–SPDP was found to be necessary to allow an interaction with the antibody.
Coupling of the C60–SPDP to the antibody (for ratios of 1 ∶ 1, 5 ∶ 1 and 10 ∶ 1) was then accomplished by reacting ZME-018 with 2-iminothiolane, which added an average of five thiol groups to the Fc fragment,24 each of which can form a new disulfide bond with the SPDP sidearm of C60–SPDP (Scheme 1). The coupling was performed in a salt solution to minimize fullerene aggregation.21d Products were purified by size-exclusion chromatography and then examined by transient absorption spectroscopy (ESI†). As shown in Fig. 2a, the C60 core's 690 nm T–T spectral signature was clearly present with intensities reflecting the reactant ratio. However, it was unclear whether covalent bonds had formed between C60–SPDP and ZME-018. Therefore, the related water-soluble C60–Ser derivative (Fig. 1b),2b was substituted for C60–SPDP in the reaction schemes with ZME-018 (10 ∶ 1 C60–Ser ∶ ZME-018). To our surprise, results for the C60–Ser derivative mirrored those of C60–SPDP. This implies that C60–(ZME-018) conjugate formation may not require covalent bond formation.
 | ||
| Fig. 2 (a) T–T spectrum of C60–SPDP–(ZME-018) immunoconjugate prepared with three different ratios of fullerene to antibody, after chromatographic purification. (b) UV absorption spectra of 0.40 µM ZME-018, the C60–SPDP–(ZME-018) immunoconjugate (chromatographically purified), and an unreacted mixture of the two components. | ||
 | ||
| Scheme 1 Schematic representation showing the formation of the C60 immunoconjugate from C60–SPDP (C60 and antibody figures not to scale). | ||
Our quantitative characterization began with BioRad protein assays, which showed that the concentration of ZME-018 in the chromatographically purified samples was 0.40 µM for C60–SPDP–(ZME-018) and 0.36 µM for C60–Ser–(ZME-018) (see ESI†). To find the corresponding fullerene concentrations in these conjugates, we used UV-vis spectroscopy. At 440 nm, the molar absorptivity of C60–Ser far exceeds that of ZME-018. The conjugate's measured 440 nm absorbance (ESI†) directly showed a C60–Ser concentration of 15 µM, implying that the ratio (C60–Ser) ∶ (ZME-018) was 42 ∶ 1.25 Spectral analysis of the C60–SPDP–(ZME-18) conjugate was more complex because absorption bands of C60–SPDP at 440 nm are not intense enough for determining concentrations <20 µM and at lower wavelengths (<350 nm) there is an overlap of absorption bands from the antibody. To account for this, we first prepared a reference solution containing only 0.40 µM ZME-018. As shown in Fig. 2b, this solution has significant absorption at 282 nm. We then added C60–SPDP until the absorbance of the mixture near 282 nm matched that of the C60–SPDP–(ZME-18) immunoconjugate known to contain a 0.40 µM concentration of antibody. The upper traces in Fig. 2b show spectra of this mixture and the conjugate. From the amount of C60–SPDP used to prepare the matching mixture, we deduced a C60–SPDP concentration of 6 µM in the conjugate, corresponding to a (C60–SPDP) ∶ (ZME-018) molar ratio of 15 ∶ 1.
Enzyme-linked immunosorbent assay (ELISA) binding curves using antigen-positive cells as targets gave mid-points of 1.2 nM for the C60–SPDP–(ZME-018) immunoconjugate, 26 nM for the C60–Ser–(ZME-018) immunoconjugate, and 724 nM for a non-specific, isotype-matched murine IgG antibody used as a control (ESI†). Amazingly, the C60–SPDP–(ZME-018) conjugate demonstrated binding midpoints similar to the non-conjugated ZME-018 antibody (mid-point of 0.46 nM), even though 8% (by weight) of the immunoconjugate is fullerene. However, the non-covalently bound C60–Ser–(ZME-018) conjugate, consisting of 17% (by weight) fullerene, exhibited a much lower affinity than C60–SPDP–(ZME-018). Regardless, the C60–Ser–(ZME-018) conjugate was still a factor of 30 more effective in binding the target than was the control.
To visualize the two C60 immunoconjugates, TEM images of both were obtained on a lacey carbon grid. Comparative images of the ZME-018 antibody and the immunoconjugate are shown in Fig. 3 (An image of C60–SPDP–(ZME-018) and experimental details are presented in the ESI†). Fig. 3 shows that the free antibody appears to have a clear globular structure ∼60 nm in diameter, whereas the image of the C60–Ser immunoconjugate is also globular but 4–5 times larger in diameter. In addition, the C60–Ser immunoconjugate image reveals numerous dark spots scattered throughout the structure that can be attributed to small aggregates of C60–Ser, ∼2–5 nm in diameter. The larger C60–Ser–(ZME-018) size may reflect disruption of hydrogen bonding networks inside the antibody or some aggregation effect.
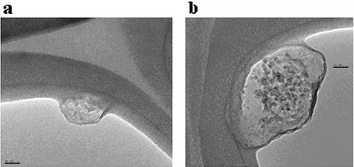 | ||
| Fig. 3 TEM images of (a) ZME-018 antibody and (b) C60–Ser–(ZME-018) immunoconjugate. The magnification is the same for both frames; scale bar length is 20 nm. The solid curved feature in the image is the lacey carbon grid material. | ||
The above experiments confirm that covalent bond formation is not necessary to form immunoconjugates of water-soluble C60 derivatives with an antibody, and that antibody to antigen binding is not significantly reduced for high C60 ∶ antibody molar ratios (15 ∶ 1). Future studies will explore the cancer cell biology of these new C60 immunoconjugates, as well as immunoconjugates derived from other fullerene-based nanostructures that have the potential for targeted imaging and therapy in medicine.11,13,26,27
At Rice University this research was supported by the Welch Foundation (grants C-0627 and C-0807), C Sixty, Inc., Carbon Nanotechnologies, Inc., and the NSF (grant CHE-0314270).
Notes and references
- Reviews: (a) A. W. Jensen, S. R. Wilson and D. I. Schuster, Bioorg. Med. Chem., 1996, 4, 767–779 CrossRef CAS; (b) L. J. Wilson, Interface, 1999, 8, 24–28 Search PubMed; (c) T. Da Ros and M. Prato, Chem. Commun., 1999, 663–669 RSC.
- (a) I. C. Wang, L. A. Tai, D. D. Lee, P. P. Kanakamma, C. K-F. Shen, T-Y. Luh, C. H. Cheng and K. C. Hwang, J. Med. Chem., 1999, 42, 4614–4620 CrossRef CAS; (b) T. Wharton, V. U. Kini, R. A. Mortis and L. J. Wilson, Tetrahedron Lett., 2001, 42, 5159–5162 CrossRef CAS; (c) A. Bar-Shir, Y. Engel and M. Gozin, J. Org. Chem., 2005, 70, 2660–2666 CrossRef CAS.
- S. Foley, C. Crowley, M. Smaihi, C. Bonfils, B. Erlanger, P. Seta and C. Larroque, Biochem. Biophys. Res. Commun., 2002, 294, 116–119 CrossRef CAS.
- E. Nakamura, H. Isobe, N. Tomita, M. Sawamura, S. Jinno and H. Okayama, Angew. Chem., Int. Ed., 2000, 39, 4254–4257 CrossRef CAS.
- C. M. Sayes, J. D. Fortner, W. Guo, D. Lyon, A. M. Boyd, K. D. Ausman, Y. J. Tao, B. Sitharaman, L. J. Wilson, J. B. Hughes, J. L. West and V. L. Colvin, Nano Lett., 2004, 4, 1881–1887 CrossRef CAS.
- (a) L. L. Dugan, D. M. Turetsky and C. Du, Proc. Natl. Acad. Sci. U. S. A., 1997, 17, 9434–9439 CrossRef; (b) L. L. Dugan, E. Lovett, C. R. Almli, T-S. Lin and D. W. Choi, Proc. Electrochem. Soc., 1998, 8, 1236–1241.
- R. Sijbesma, G. Srdanov, F. Wudl, J. A. Castoro, C. Wilkins, S. H. Friedman, D. L. DeCamp and G. L. Kenyon, J. Am. Chem. Soc., 1993, 115, 6510–6512 CrossRef CAS.
- (a) K. A. Gonzalez, L. J. Wilson, W. Wu and G. H. Nancollas, Bioorg. Med. Chem., 2002, 10, 1991–1997 CrossRef CAS; (b) A. L. Mirakyan and L. J. Wilson, J. Chem. Soc., Perkin Trans. 2, 2002, 1173–1176 RSC.
- T. Wharton and L. J. Wilson, Tetrahedron Lett., 2002, 43, 561–564 CrossRef CAS.
- (a) Y. Yamakoshi, N. Umezawa, A. Ryu, K. Arakane, N. Miyata, Y. Goda, T. Masumizu and T. Nagano, J. Am. Chem. Soc., 2003, 125, 12803–12809 CrossRef CAS; (b) C. Yu, T. Canteenwala, M. E. El-Khouly, Y. Araki, K. Pritzker, O. Ito, B. C. Wilson and L. Y. Chiang, J. Mater. Chem., 2005, 15, 1857–1864 RSC.
- T. Y. Zakharian, A. Seryshev, B. Sitharaman, B. E. Gilbert, V. Knight and L. J. Wilson, J. Am. Chem. Soc., 2005, 127, 12508–12509 CrossRef CAS.
- D. W. Cagle, T. P. Thrash, M. Alford, L. P. F. Chibante, L. J. Ehrhardt and L. J. Wilson, J. Am. Chem. Soc., 1996, 118, 8043–8047 CrossRef CAS.
- (a) M. Mikawa, H. Kato, M. Okumura, M. Narazaki, Y. Kanazawa, N. Miwa and H. Shinohara, Bioconjugate Chem., 2001, 12, 510–514 CrossRef CAS; (b) H. Kato, Y. Kanazawa, M. Okumura, A. Taninaka, T. Yokawa and H. Shinohara, J. Am. Chem. Soc., 2003, 125, 4391–4397 CrossRef CAS; (c) R. D. Bolskar, A. F. Benedetto, L. O. Husebo, R. E. Price, E. F. Jackson, S. Wallace, L. J. Wilson and J. M. Alford, J. Am. Chem. Soc., 2003, 125, 5471–5478 CrossRef CAS; (d) B. S. Sitharaman, R. D. Bolskar, I. Rusakova and L. J. Wilson, Nano Lett., 2004, 4, 2373–2378 CrossRef CAS; (e) É. Tóth, R. D. Bolskar, A. Borel, G. González, L. Helm, A. E. Merbach, B. Sitharaman and L. J. Wilson, J. Am. Chem. Soc., 2005, 127, 799–805 CrossRef CAS.
- M. Kellermann, W. Bauer, A. Hirsch, B. Schade, K. Ludwig and C. Böttcher, Angew. Chem., 2004, 116, 3019–3022 ( Angew. Chem., Int. Ed. , 2004 , 43 , 2959–2962 ) CrossRef.
- (a) A. Casadevall, E. Dadachova and L. Pirofski, Nat. Rev. Microbiol., 2004, 2, 695–703 Search PubMed; (b) A. Wu and P. D. Senter, Nat. Biotechnol., 2005, 23, 1137–1146 CrossRef CAS; (c) G. P. Adams and L. Weiner, Nat. Biotechnol., 2005, 23, 1147–1157 CrossRef CAS.
- R. R. Kantor, A. K. Ng, P. Giacomini and S. Ferrone, Hybridoma, 1982, 1, 473–482 CAS.
- (a) D. J. Macey, S. J. Denardo, G. L. Denardo, J. K. Goodnight and M. W. Unger, Am. J. Physiol. Imaging, 1988, 3, 1–6 Search PubMed; (b) M. Koizumi, K. Endo, Y. Watanabe, T. Saga, H. Sakahara and J. Konishi, Jpn. J. Cancer Res., 1988, 79, 973–981 CAS.
- M. G. Rosenblum, L. H. Cheung, Y. Liu and J. W. Marks, Cancer Res., 2003, 63, 3995–4002 CAS.
- J. M. Kirkwood, R. D. Neumann, S. S. Zoghbi, M. S. Ernstoff, E. A. Cornelius, C. Shaw, T. Ziyadeh, J. A. Fine and M. W. Unger, J. Clin. Oncol., 1987, 8, 1247–1255.
- (a) M. G. Rosenblum, J. L. Murray, L. Cheung, R. Rifkin, S. Salmon and R. A. Bartholomew, Mol. Biotherm., 1991, 3, 6–13 Search PubMed; (b) K. Mujoo, L. Cheung, J. L. Murray and M. G. Rosenblum, Cancer Immunol. Immunother., 1995, 40, 339–345 Search PubMed.
- (a) R. A. Kotelnikova, G. N. Bogdanov, E. C. Frog, A. I. Kotelnikov, V. N. Shtolko, V. S. Romanova, S. M. Andreev, A. A. Kushch, N. E. Fedorova, A. A. Medzhidova and G. G. Miller, J. Nanopart. Res., 2003, 5, 561–566 CrossRef CAS; (b) A. P. Maierhofer, M. Brettreich, S. Burghardt, O. Vostrowsky, A. Hirsch, S. Langridge and T. M. Bayerl, Langmuir, 2000, 16, 8884–8891 CrossRef CAS; (c) M. Braun, S. Atalick, D. M. Guldi, H. Lanig, M. Brettreich, S. Burghardt, M. Matzimarinaki, E. Ravanelli, M. Prato, R. Eldik and A. Hirsch, Chem.–Eur. J., 2003, 9, 3867–3875 CrossRef CAS; (d) S. Laus, B. Sitharaman, É. Tóth, R. D. Bolskar, L. Helm, S. Asokan, M. S. Wong, L. J. Wilson and A. E. Merbach, J. Am. Chem. Soc., 2005, 127, 9368–9369 CrossRef CAS; (e) B. Belgorodsky, L. Fadeev, V. Ittah, H. Benyamini, S. Zelner, D. Huppert, A. B. Kotlyar and M. Gozin, Bioconjugate Chem., 2005, 16, 1058–1062 CrossRef CAS; (f) P. Nednoor, M. Capaccio, V. G. Gavalas, M. S. Meier, J. E. Anthony and L. G. Bachas, Bioconjugate Chem., 2004, 15, 12–15 CrossRef CAS; (g) B. C. Braden, F. A. Goldbaum, B-X. Chen, A. N. Kirschner, S. R. Wilson and B. F. Erlanger, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 12193–12197 CrossRef CAS.
- (a) K. D. Ausman and R. B. Weisman, Res. Chem. Intermed., 1997, 6, 431–451 CrossRef; (b) J. P. Mittal, Pure Appl. Chem., 1995, 67, 103–110 CrossRef CAS; (c) S. D. M. Islam, M. Fujitsuka, O. Ito, A. Ikeda, T. Hatano and S. Shinkai, Chem. Lett., 2000, 1, 78–79 CrossRef; (d) R. V. Bensasson, M. N. Berberan-Santos, M. Brettreich, J. Frederiksen, H. Göttinger, A. Hirsch, E. J. Land, S. Leach, D. J. McGarvey, H. Schönberger and C. Schröder, Phys. Chem. Chem. Phys., 2001, 3, 4679–4683 RSC.
- J. Carlsson, H. Drevin and R. Axén, Biochem. J., 1978, 173, 723–737 CAS.
- N. Watanabe, D. A. Goodwin, C. F. Meares, M. Mctigue, W. Chaovapong, C. M. Ransone and O. Renn, Cancer Res., 1994, 54, 1049–1054 CAS.
- A ratio of 40 ∶ 1 is reasonable even though the initial ratio of C60–SPDP ∶ antibody was only 10 ∶ 1 because a large amount of antibody precipitate always occurs upon immunoconjugate formation over a 24 h period.
- Y. A. Mackeyev, J. W. Marks, M. G. Rosenblum and L. J. Wilson, J. Phys. Chem. B, 2005, 109, 5482–5484 CrossRef CAS.
- B. Sitharaman, K. R. Kissell, K. B. Hartman, L. A. Tran, A. Baikalov, I. Rusakova, Y. Sun, H. A. Khant, S. J. Ludtke, W. Chiu, S. Laus, É. Toth, A. E. Merbach and L. J. Wilson, Chem. Commun., 2005, 3915–3917 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and instrumentation detail. See DOI: 10.1039/b601717g |
| This journal is © The Royal Society of Chemistry 2006 |
