Balancing supramolecular reagents for reliable formation of co-crystals†
Christer B.
Aakeröy
*,
John
Desper
and
Benjamin M. T.
Scott
Department of Chemistry, Kansas State University, Manhattan, KS 66506, USA. E-mail: aakeroy@ksu.edu
First published on 23rd February 2006
Abstract
The rational design and synthesis of a supramolecular reagent (SR) composed of two distinct hydrogen bonding sites (pyrazole–benzamide), and four co-crystals resulting from reactions between this SR and a variety of carboxylic acids are described; the observed primary intermolecular interaction is consistent and predictable in each case.
In supramolecular synthesis the desired products are typically held together by reversible intermolecular interactions, and therefore synthetic procedures normally have to take place in a one-pot process.1
A possible solution to the problem of making one-pot synthesis “sequential” may be to devise modular assembly processes based upon a hierarchy of intermolecular interactions. The hydrogen bond lends itself readily to electrostatic or geometric fine tuning, and there have consequently been numerous reports on the construction of specific motifs and architectures through selective hydrogen bond interactions.2
Recent work on isonicotinamide has demonstrated that it is a reliable supramolecular reagent (SR) which, in combination with carboxylic acids,3 produces co-crystals in a high supramolecular yield.4 In binary 1 ∶ 1 isonicotinamide/carboxylic acid co-crystals, the primary intermolecular interaction is typically the O–H⋯N hydrogen bond between the acid and the N-heterocyclic nitrogen atom (Fig. 1).
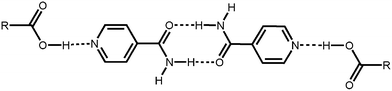 | ||
| Fig. 1 | ||
The physical basis for this behavior can be ascribed to the tendency of the system to maximise electrostatic interactions. The pyridyl nitrogen atom and the –OH moiety provide the best hydrogen bond acceptor and donor,2,5 respectively, and these moieties display a strong preference for each other. To place this hierarchical view of competitive molecular recognition events on a firmer footing, it is necessary to establish whether intermolecular interactions can be manipulated by altering the balance between the two binding sites on a ditopic SR. Unfortunately, isonicotinamide does not offer a good test bed for such studies because it is not possible to modify one binding site without affecting the other, as both are connected to the same conjugated system. Consequently, we synthesized a new SR that would allow an examination of the competition between two uncoupled binding sites.
The key to understanding and predicting the supramolecular behavior of this family of SRs is the relative hydrogen bonding ability (based on basicity and molecular electrostatic surface potential) of the N-heterocycle. If the pyridyl site is replaced with a more basic moiety such as benzimidazole (while retaining the amide as the second binding site), an incoming carboxylic acid will again bind to the N-heterocycle rather than to the amide.6 On the other hand, if the basicity of the nitrogen atom is lowered sufficiently, the resulting O–H⋯N hydrogen bond should at some point become so weak that the carboxylic acid abandons the N-heterocycle in favor of an acid⋯amide dimeric interaction.7
To test this hypothesis, we synthesized a new ditopic SR, 4-[(pyrazol-1-yl)methyl]-benzamide, and allowed it to react with a series of carboxylic acids.8 The calculated molecular electrostatic potential surfaces9 of three relevant ditopic SRs (1: isonictotinamide, 2: 4-[(benzimidazol-1-yl)methyl]-benzamide and 3: 4-[(pyrazol-1-yl)methyl]-benzamide) and their pKa values10 are shown in Fig. 2.
 | ||
| Fig. 2 | ||
The two binding sites on 4-[(pyrazol-1-yl)methyl]-benzamide are separated by a methylene bridge, which allows for their independent tuning by the use of electron donating/withdrawing groups. The increased flexibility of the molecule also enhances solubility, which typically facilitates the solution-based synthesis of co-crystals.
The crystal structure determination of 1 shows that a binary 2 ∶ 1 co-crystal has been prepared, as intended.11 Two symmetry related acid⋯amide hydrogen bonds (N⋯O 2.515(2) Å and O⋯O 2.932(3) Å) drive the assembly of this co-crystal and create the primary trimeric supermolecule (Fig. 3). Adjacent trimers are organized through long N–H⋯O contacts (ca. 3.26 Å) between the anti proton of the amide and an –OH moiety on a neighboring supermolecule.
 | ||
| Fig. 3 A trimeric supermolecule in the 2 ∶ 1 binary co-crystal 1 (50% probability level). Oxalic acid (located on an inversion center; symmetry related atoms at −x − 1, −y, −z) interacts with the amide moiety instead of with the pyrazole moiety. | ||
The crystal structure determination of 2 shows that a binary 2 ∶ 1 co-crystal formed, as expected.12 The primary intermolecular interaction in 2 is the heteromeric O–H⋯O/O⋯H–N acid⋯amide motif (O⋯O 2.586(2) Å and N⋯O 2.868(2) Å). Symmetry related acid⋯amide synthons generate the central trimeric supermolecule (Fig. 4). Adjacent supermolecules are interconnected through a secondary N–H⋯N interaction between the anti proton of the amide and the free pyrazole nitrogen atom (N⋯N 2.978(2) Å), and oriented perpendicular to each other.
 | ||
| Fig. 4 A trimeric supermolecule is the supramolecular centre piece in the 2 ∶ 1 binary co-crystal 2 (50% probability level). Succinic acid (located on an inversion centre; symmetry related atoms at −x + 2, −y + 3, −z) binds to the amide moiety. | ||
3 is a co-crystal composed of benzoic acid and 4-[(pyrazol-1-yl)methyl]-benzamide in the expected 1 ∶ 1 ratio,13 with two inequivalent dimeric supermolecules constructed from heteromeric acid⋯amide dimers (N⋯O 2.9740(15) Å and O⋯O 2.5692(13) Å; N⋯O 2.8599(15) Å and O⋯O 2.5702(13) Å) (Fig. 5). Each dimer is linked to an adjacent symmetry related dimer through an N–H⋯N interaction between the anti proton of the amide and the free pyrazole nitrogen atom (N⋯N 2.9946(16) and 2.9840(15) Å).
 | ||
| Fig. 5 Two unique supermolecular dimers in the 1 ∶ 1 binary co-crystal 3 (50% probability level). The carboxylic acids interact directly with the amide moieties. | ||
The structure determination of 4 shows a co-crystal of the SR and 2-fluorobenzoic acid in a 1 ∶ 1 ratio.14 The driving force for the construction of this co-crystal is the amide⋯acid heteromeric dimer (N⋯O 2.879(2) and O⋯O 2.5415(17) Å) (Fig. 6). Adjacent dimers are linked through an N–H⋯N interaction between the anti proton of the amide and the free pyrazole nitrogen atom of an adjacent dimer (2.954(2) Å).
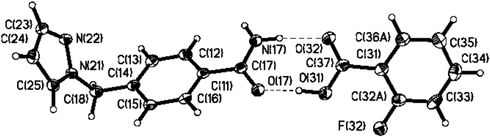 | ||
| Fig. 6 A dimeric supermolecule in the 1 ∶ 1 binary co-crystal of 4 (50% probability level) generated through a heteromeric acid⋯amide synthon. | ||
All four structures display the same principle motif; the ditopic pyrazole/benzamide SR consistently binds to a neighbouring carboxylic acid via its amide moiety.15 This supramolecular behaviour is in stark contrast with the interactions that have been observed between carboxylic acids and SRs composed of an amide moiety and substantially more basic N-heterocyclic nitrogen atoms than the pyrazole moiety (e.g. pyridine or benzimidazole).
These results demonstrate that it is possible to move and reorganize molecules within supramolecular aggregates by following relatively simple design principles based on solution-based pKa-values or calculated molecular electrostatic potential surfaces. Even though concepts such as pKa values do not translate into bond strengths or free-energies of complexation, they are clearly valuable supramolecular indicators when applied to members of the same functional group class in a systematic manner.
This study also supports the idea that a hierarchical view of intermolecular interactions can provide a foundation for effective and versatile synthetic supramolecular strategies, because hydrogen bond strengths of specific molecular building blocks can be carefully tuned through relatively simple covalent modifications. Thus, by modifying the electrostatic nature of individual binding sites by covalent means, it should be possible to refine supramolecular synthesis to the point where we can construct a wide variety of heteromolecular architectures with predetermined connectivities and dimensions.
We are grateful for financial support from NSF (CHE-0316479).
Notes and references
- Some elegant examples of sequential “crystal engineering” have been reported: S. Ferlay and W. Hosseini, Chem. Commun., 2004, 788 Search PubMed; J. C. MacDonald, P. C. Dorrestein, M. M. Pilley, M. M. Foote, J. L. Lundburg, R. W. Henning, A. J. Schultz and J. L. Manson, J. Am. Chem. Soc., 2000, 122, 11692 RSC.
- C. B. Aakeröy, J. Desper and J. F. Urbina, Chem. Commun., 2005, 2820 RSC; C. B. Aakeröy and D. J. Salmon, CrystEngComm, 2005, 7, 439 RSC; T. R. Shattock, P. Vishweshwar, Z. Wang and M. J. Zaworotko, Cryst. Growth. Des., 2005, 5, 2046 CrossRef CAS; C. B. Aakeröy, J. Desper and J. F. Urbina, CrystEngComm, 2005, 31, 193 RSC; Ö. Almarsson and M. J. Zaworotko, Chem. Commun., 2004, 1889 RSC; D. Braga and F. Grepioni, Chem. Commun., 2005, 3635 RSC; P. Vishweshwar, J. A. McMahon, M. L. Peterson, M. B. Hickey, T. R. Shattock and M. J. Zaworotko, Chem. Commun., 2005, 4601 RSC; B. K. Saha, A. Nangia and M. Jaskolski, CrystEngComm, 2005, 335 Search PubMed; G. R. Desiraju, Acc. Chem. Res., 2002, 35, 565 CrossRef CAS; B. R. Bhogala, S. Basavoju and A. Nangia, Cryst. Growth. Des., 2005, 5, 1683 CrossRef CAS; M. W. Hosseini, CrystEngComm, 2004, 6, 318 RSC; A. V. Trask, S. W. D. Motherwell and W. Jones, Chem. Commun., 2004, 890 RSC; A. V. Trask, J. van de Streek, S. W. D. Motherwell and W. Jones, Cryst. Growth. Des., 2005, 5, 2233 CrossRef CAS; C. B. Aakeröy, A. M. Beatty and B. A. Helfrich, Angew. Chem., Int. Ed., 2001, 40, 3240 CrossRef CAS; S. Varughese and V. R. Pedireddi, Chem.–Eur. J, 2006, 12, 1597 CrossRef.
- C. B. Aakeröy, A. M. Beatty and B. A. Helfrich, J. Am. Chem. Soc., 2002, 124, 14425 CrossRef.
- In the Cambridge Structural Database (v. 5.27) there are over 25 binary isonicotinamide–carboxylic acid co-crystals. The acid–py hydrogen bond is present in over 90% of those structures, corresponding to a high supramolecular yield (frequency of occurrence of desired motif).
- M. C. Etter, Acc. Chem. Res., 1990, 23, 120 CrossRef CAS; M. C. Etter, J. Phys. Chem., 1991, 95, 4601 CrossRef CAS.
- C. B. Aakeröy, J. Desper and J. F. Urbina, unpublished results.
- Replacing the pyridyl moiety with a phenyl backbone increases the negative potential on the carbonyl oxygen atom of the attached –C(
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)NH2 group, which would also favor binding at the amide site. However, the primary difference between benzimidazole–benzamide and benzimidazole–pyrazole SRs is the electrostatic potential around the available heterocyclic nitrogen atom.
O)NH2 group, which would also favor binding at the amide site. However, the primary difference between benzimidazole–benzamide and benzimidazole–pyrazole SRs is the electrostatic potential around the available heterocyclic nitrogen atom. - 4-(1H-1-pyrazolylmethyl)benzamide, oxalic acid (2 ∶ 1) (1): 4-(1H-1-pyrazolylmethyl)benzamide (25 mg, 0.124 mmol) and oxalic acid (11 mg, 0.124 mmol) were added to a test tube along with acetonitrile. The mixture was heated gently until components were in solution. Colourless plates formed after 4 d; mp: 143–145 °C. 4-(1H-1-pyrazolylmethyl)benzamide, succinic acid (2 ∶ 1) (2): 4-(1H-1-pyrazolylmethyl)benzamide (25 mg, 0.124 mmol) and succinic acid (15 mg, 0.124 mmol) were added to a test tube along with acetonitrile. The mixture was heated gently until components were in solution. Colourless prisms formed after 4 d; mp: 145–150 °C. 4-(1H-1-pyrazolylmethyl)benzamide, benzoic acid (1 ∶ 1) (3): 4-(1H-1-pyrazolylmethyl)benzamide (25 mg, 0.124 mmol) and benzoic acid (14 mg, 0.124 mmol) were added to a test tube along with acetonitrile. The mixture was heated gently until components were in solution. Colourless prisms formed after 4 d; mp: 100 °C. 4-(1H-1-pyrazolylmethyl)benzamide, 2-fluorobenzoic acid (1 ∶ 1) (4): 4-(1H-1-pyrazolylmethyl)benzamide (25 mg, 0.124 mmol) and 2-fluorobenzoic acid (14 mg, 0.124 mmol) were added to a test tube along with acetonitrile. The mixture was heated gently until components were in solution. Colourless prisms formed after 4 d; mp: 108–110 °C.
- The three SRs shown in Fig. 2 were constructed using Spartan ’04 (Wavefunction, Inc. Irvine, CA). Their molecular geometries were optimized using AM1, and the maxima and minima of the molecular electrostatic potential surface (0.002 e/au isosurface) were determined using a positive point charge in a vacuum as the probe.
- pKa values were obtained through calculations of the conjugate acids using ACD/Solaris v. 4.76, Advanced Chemistry Development, Inc., Toronto, ON, Canada, 1994–2005, http://www.acdlabs.com Search PubMed.
- Crystal data for 4-(1H-1-pyrazolylmethyl)benzamide, oxalic acid (2 ∶ 1) (1): C24H24N6O6, M = 492.49, monoclinic, space group P2(1)/n, a = 4.2359(8), b = 49.029(9), c = 5.5087(11), α = 90.00, β = 92.780(4), γ = 90.00°, V = 1142.7(4) Å3, Z = 2, Dc = 1.431 g cm−3, μ(Mo-Kα) = 0.106 mm−1, crystal size 0.34 × 0.22 × 0.01 mm. Data were collected at 100 K on a Bruker SMART 1000 diffractometer using Mo-Kα radiation. A total of 8069 reflections (2.49° < θ < 28.39°) were processed, of which 2435 were unique and significant with I > 2σ(I). Structure solution and refinement were carried out SHELXS-97 and SHELXL-97.16Rint = 0.0362. Final residuals for I > 2σ(I) were R1 = 0.0735 and wR2 = 0.1529 (GOF = 1.253). CCDC 292716. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b517118k.
- Crystal data for 4-(1H-1-pyrazolylmethyl)benzamide, succinic acid (2 ∶ 1) (2): C26H28N6O6, M = 520.54, monoclinic, space group P2(1)/n, a = 9.5363(9), b = 7.3143(6), c = 17.6580(15) Å, α = 90.00, β = 96.130(7), γ = 90.00°, V = 1224.63(19) Å3, Z = 2, Dc = 1.412 g cm−3, μ(Mo-Kα) = 0.103 mm−1, crystal size 0.40 × 0.35 × 0.25 mm. Data were collected at 100 K on a Bruker SMART 1000 diffractometer using Mo-Kα radiation. A total of 8075 reflections (2.32° < θ < 27.85°) were processed, of which 2042 were unique and significant with I > 2σ(I). Structure solution and refinement were carried out SHELXS-97 and SHELXL-97.16Rint = 0.1114. Final residuals for I > 2σ(I) were R1 = 0.0639 and wR2 = 0.1686 (GOF = 1.067). CCDC 292717. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b517118k.
- Crystal data for 4-(1H-1-pyrazolylmethyl)benzamide, benzoic acid (1 ∶ 1) (3): C18H17N3O3, M = 323.35, triclinic, space group P-1, a = 9.0434(8), b = 10.5552(9), c = 17.4323(15) Å, α = 101.8070(10), β = 102.1260(10), γ = 94.3700(10)°, V = 1580.1(2) Å3, Z = 4, Dc = 1.359 g cm−3, μ(Mo-Kα) = 0.095 mm−1, crystal size 0.28 × 0.28 × 0.15 mm. Data were collected at 100 K on a Bruker SMART 1000 diffractometer using Mo-Kα radiation. A total of 14134 reflections (1.99° < θ < 28.27°) were processed, of which 5988 were unique and significant with I > 2σ(I). Structure solution and refinement were carried out SHELXS-97 and SHELXL-97.16Rint = 0.0270. Final residuals for I > 2σ(I) were R1 = 0.0438 and wR2 = 0.1184 (GOF = 1.058). CCDC 292718. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b517118k.
- Crystal data for 4-(1H-1-pyrazolylmethyl)benzamide, 2-fluorobenzoic acid (1 ∶ 1) (4): C18H16FN3O3, M = 341.34, monoclinic, space group P2(1)/n, a = 4.3453(7), b = 10.8147(17), c = 34.003(5) Å, α = 90.00, β = 90.541(2), γ = 90.00°, V = 1597.8(4) Å3, Z = 4, Dc = 1.419 g cm−3, μ(Mo-Kα) = 0.106 mm−1, crystal size 0.14 × 0.20 × 0.35 mm. Data were collected at 100 K on a Bruker SMART 1000 diffractometer using Mo-Kα radiation. A total of 9978 reflections (1.98° < θ < 26.01°) were processed, of which 2486 were unique and significant with I > 2σ(I). Structure solution and refinement were carried out using SHELXS-97 and SHELXL-97.16Rint = 0.0362. Final residuals for I > 2σ(I) were R1 = 0.0407 and wR2 = 0.1053 (GOF = 1.034). CCDC 292719. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b517118k.
- The secondary N–H⋯N interaction, which is observed in 2–4, does not directly facilitate the assembly of the co-crystals as it does not involve the carboxylic acid.
- G. M. Sheldrick, SHELXS-97, Program for solution of crystal structures, University of Göttingen, Germany, 1997 Search PubMed; G. M. Sheldrick, SHELXL-97, Program for refinement of crystal structures, University of Göttingen, Germany, 1997 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: Synthesis of supramolecular reagents. See DOI: 10.1039/b517118k |
| This journal is © The Royal Society of Chemistry 2006 |
