Rapid trace detection of triacetone triperoxide (TATP) by complexation reactions during desorption electrospray ionization†
Ismael Cotte-Rodríguez, Hao Chen and R. Graham Cooks*
Department of Chemistry, 560 Oval Drive, Purdue University, West Lafayette, IN 47907, USA. E-mail: cooks@purdue.edu; Fax: (765) 494-9421; Tel: (765) 494-5263
First published on 12th January 2006
Abstract
Desorption electrospray ionization (DESI) mass spectrometry is used for rapid, specific and sensitive detection of trace amounts of the notorious explosive TATP present on ambient surfaces by alkali metal complexation in a simple spray technique.
Incidents involving the use of the cyclic peroxide-based explosive, triacetone triperoxide (TATP) continue,1–3 the latest being the bombing of London subway trains.4 TATP has become an illicit explosive of choice due to its straightforward synthesis using readily available precursor chemicals, acetone and hydrogen peroxide.2,3,5–8 First synthesized by Wolffenstein in 1895,9 this peroxide-based explosive is a nine-membered cyclic structure, known for its extreme sensitivity to detonation on heating, impact or friction.10–13 It also has explosive power comparable to TNT.10,11
TATP detection represents a serious challenge because conventional explosives detection devices (EDDs), such as those used at airports around the world, rely on the presence of nitro groups or metallic elements for a positive response.8,14,15 In addition, TATP has no significant absorption in the ultraviolet region and does not exhibit fluorescence. As a result, its detection has been limited to infrared and Raman spectroscopy, enzyme-based tests16 and mass spectrometry (MS).2,3,8,15 Each of these methods suffers from serious drawbacks such as low sensitivity and/or slow response. The best available methods involve liquid chromatography (LC) coupled with electrospray ionization (ESI) MS or atmospheric pressure chemical ionization (APCI) MS and LC with fluorescence detection;7,15 they still require sample handling, take time for the chromatographic separation and tend to show excessive fragmentation in the mass spectra.
All these facts indicate a strong need for an instrumental method for detection of TATP and other organic peroxides that can be performed in-situ, without any sample pre-treatment, with high sensitivity and selectivity in short analysis times, and without excessive fragmentation of the fragile TATP molecule. The recently developed method of desorption electrospray ionization (DESI)17–19 meets all these requirements. DESI is based on directing a pneumatically-assisted electrospray onto a surface and collecting the secondary ions generated by the interaction of charged microdroplets with analyte molecules on the surface.17
We show DESI to be a sensitive and selective ionization method for detecting trace amounts of the peroxide-based explosive TATP on ambient surfaces. This is facilitated by the formation of complexes with the alkali metal ions (Li+, Na+ and K+). Collision-induced dissociation (CID) of the TATP–metal complexes occurs with retention of the metal in the fragment ions. Labeling experiments and density functional theory (DFT) calculations helped elucidate the fragmentation mechanism.
Reactive-DESI19 experiments were performed using, as the spray solvent, methanol/water solutions doped with ammonium acetate (CH3COONH4), sodium chloride (NaCl), lithium chloride (LiCl) or potassium chloride (KCl), in order to enhance the selectivity and instrumental response to peroxide explosives. Source parameters are summarized elsewhere.19 The positive ions formed were sampled using a Thermo Finnigan LTQ mass spectrometer. TATP was synthesized as described in the literature20 and it showed a melting point of 97–98 °C, consistent with reported values.3,6 Limits of detection (LOD) for TATP on different surfaces, in the presence or absence of matrices, are summarized in Table 1 (note that LOD refers to the total amount of analyte present on the surface and to a 3 ∶ 1 signal-to-noise ratio). Peroxide samples were deposited on paper, metal or brick in an area of 1 cm2 of which some 4 mm2 was typically sampled.
| Surface | Matrix | Ion observed | LOD | S/N at LOD |
|---|---|---|---|---|
| Paper | Methanol | (M + Na)+ | 1 ng | 4 ∶ 1 |
| Diesel | (M + Na)+ | 10 ng | 3 ∶ 1 | |
| WD-40 | (M + Na)+ | 10 ng | 3 ∶ 1 | |
| Windex | (M + Na)+ | 10 ng | 3 ∶ 1 | |
| Vinegar | (M + Na)+ | 20 ng | 3 ∶ 1 | |
| Brick | Methanol | (M + Na)+ | 2 ng | 3 ∶ 1 |
| Diesel | (M + Na)+ | 20 ng | 4 ∶ 1 | |
| WD-40 | (M + Na)+ | 20 ng | 4 ∶ 1 | |
| Windex | (M + Na)+ | 20 ng | 3 ∶ 1 | |
| Vinegar | (M + Na)+ | 50 ng | 3 ∶ 1 | |
| Metal | Methanol | (M + Na)+ | 1 ng | 3 ∶ 1 |
Fig. 1 shows the positive ion DESI mass spectrum of a 10 ng TATP sample deposited on paper and then sprayed using CH3COONH4 (10 mM) and NaCl (10 mM) in methanol/water (70 ∶ 30). Only a small fraction of the sample was interrogated by the DESI spray.19 The most significant ions in the positive ion DESI spectrum are observed at m/z 240, m/z 245 and m/z 223, and they correspond to (TATP + NH4)+, (TATP + Na)+ and (TATP + H)+, respectively. Tandem mass spectrometry was used to confirm the identity of these ions. For example, the ion of m/z 240 undergoes a characteristic loss of 17 mass units corresponding to NH3 to give a fragment ion at m/z 223 (TATP + H)+ (8% relative abundance). The CID fragmentation mechanism for the alkali metal complexes was different from that of the ammonia adduct. The (TATP + Na)+ complex at m/z 245 retained the metal ion under CID conditions, giving a major product ion at m/z 215 (C7H12O6 + Na)+ probably due to loss of ethane (see proposed CID fragmentation mechanism below).
 | ||
| Fig. 1 Positive ion DESI spectrum of 10 ng TATP deposited on 1 cm2 of paper. Inset (left side) is a calibration curve for TATP from 1–5000 ng monitoring the fragmentation of the ion of m/z 245 to give m/z 215. The MS/MS product ion spectrum of (TATP + Na)+, m/z 245, is shown in the inset (right side). | ||
When TATP was analyzed by other ionization techniques including ESI, APCI or electrosonic spray ionization (ESSI),21 no (M + H)+, (M − H)+ or (M + metal)+ ions were observed. However, DESI yields ions of the type (M + H)+ and alkali metal adducts, (M + metal)+. This shows the gentle character of the DESI method for the ionization of this extremely fragile molecule. The experiment showed low LOD's, for example, using NaCl as dopant in the solvent spray, the LOD for this peroxide-based explosive was 1 ng TATP, where this refers to the total amount deposited on paper or metal, and 2 ng on brick (Table 1). The LODs observed are comparable to those obtained with other commonly used techniques7,15,22–24 (e.g., LOD of 3.3 ng by LC/MS24 and LOD of 6.4 ng by GC/MS23), but the method has the advantage of rapid detection without sample preparation.
Quantification on paper was performed using an eight-point calibration curve covering a linear dynamic range of more than three orders of magnitude (1 to 5000 ng) by depositing sample in an area of 1 cm2 (Fig. 1, left inset). The absolute abundance in single reaction monitoring (SRM) of the (TATP + Na)+ complex (m/z 245 → m/z 215) was used to prepare the multi-point calibration curve. Triplicate measurements showed an RSD of less than 3% (calibration curve: y = 23.494x + 461.68, R2 = 0.9998). Using this calibration curve, an “unknown” TATP sample on paper was analyzed for TATP content. The average absolute abundance for triplicate measurements of the m/z 245 ion yielded a value of 1017 ng from the calibration curve when the actual total amount of TATP deposited on paper was 1000 ng, providing a rough idea of the accuracy of this measurement.
The performance of the DESI method in the detection of TATP in complex matrices such as diesel, vinegar, Windex, and WD-40 was evaluated by depositing the mixture containing TATP onto paper or brick surfaces, the latter being a greater challenge due to its porosity. LOD's for TATP detected in several matrices and deposited onto paper or brick are summarized in Table 1 (additional information regarding the performance of the DESI method in the detection of TATP in complex matrices is provided in the Supporting Information).
In order to rationalize the fragmentation route observed for the alkali metal complexes of TATP and interpret the CID spectra, we propose a mechanism that is consistent with the observed data and with separate labelling experiments. The first step proposed in the CID fragmentation mechanism is the homolytic cleavage of the fragile peroxide bond O–O (34–37 kcal/mol)5,25 to form a biradical (Scheme 1a). Subsequently, rearrangement takes place by two methyl shifts from adjacent carbons to the oxygen radical sites. Finally, the methyl groups are released after homolysis of the O–CH3 bond, driven by their close proximity which allows recombination to produce ethane, viz. the ion at m/z 245 fragments to give a product ion at m/z 215 (C7H12O6 + Na)+.
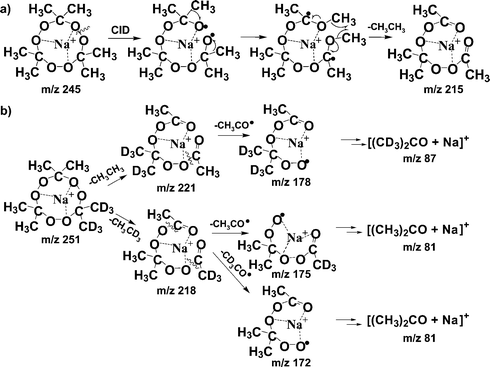 | ||
| Scheme 1 Proposed mechanism for dissociation of (a) the (TATP + Na)+ complex at m/z 245 and (b) the deuterated complex (TATPd6 + Na)+ at m/z 251. | ||
The ion at m/z 215 suffers consecutive fragmentations corresponding to loss of one CH3CO radical giving rise to the product ion at m/z 172 (C5H9O5 + Na)+ (Fig. 1, right side) while further fragmentation of the m/z 172 ion produces (C3H6O + Na)+ at m/z 81. The retention of the sodium ion during CID of the (TATP + Na)+ complex is remarkable, suggesting the existence of a strong interaction between TATP and sodium. This result is analogous to the observation of prior cleavage of a diazo bond to form carbenes by loss of N2 and retention of non-covalently bound peptide/copper ion in the “molecular mousetrap” complex ions reported by Beauchamp et al.26,27
Isotopic labeling experiments support the proposed fragmentation mechanism. Fig. 2 shows the DESI-generated sodium complex ions from labeled TATPs (TATP-dn, n = 6, 12, and 18, deuterium labeled terminal methyl groups) and two typical CID spectra. The expected isotope-labeled fragment ions were observed in the CID spectra (Figs. 2b and 2c). The abundance ratios of the observed isotope-labeled fragment ions are in agreement with the proposed fragmentation pathway (Scheme 1b), as described in the Supporting Information.† Similar fragmentation behavior was observed for TATP complexes with K+ and Li+. In addition, tetracetone tetraperoxide (TrATrP), the tetramer of acetone peroxide, behaved analogously to TATP in the fragmentation reactions of its Na+, K+, and Li+ cationized forms. (For the complete CID experimental data including Na+, K+, Li+, and NH4+ complexes with labeled TATP and TrATrP, refer to Supporting Information).
 | ||
| Fig. 2 (a) Positive ion DESI spectrum of 0.7 µg of a mixture of labeled TATP (d6 up to d18) deposited on 1 cm2 of metal surface. Methanol/water (70 ∶ 30) doped with NaCl (10 mM) was used as the spray solvent and a 4 mm2 area was sampled. The corresponding MS/MS product ion spectra of (TATP-d6 + Na)+, m/z 245, and (TATP-d18 + Na)+, m/z 263, are shown in (b) and (c), respectively. | ||
In a separate experiment, the spray solvent (methanol/water) was doped with Na+, K+, Li+ and NH4+ and sprayed onto a metal surface containing TATP to examine the selective detection of TATP by forming stable complexes with multiple dopants. This approach can be used in combination with tandem mass spectrometry, and provides another tool to help confirm the presence of TATP. (For more information on the experimental procedures and spectra, refer to Supporting Information).
Density functional theory (DFT) calculations were performed in order to calculate the binding energies for the Na+ complex with TATP. Fig. 3 displays the optimized structures for TATP and (TATP + Na)+ at the level B3LYP/6-31G.
 | ||
| Fig. 3 DFT calculated structures at the level of B3LYP/6-31G for (a) TATP, (b) (TATP + Na)+, (c) side view of the (TATP + Na)+ complex. | ||
The Na+–TATP binding energy was found to be 47 kcal/mol, ∼ 11 kcal/mol higher than the energy of the weak O–O (36.3 kcal/mol)5 bond. This can account for the retention of the sodium ion during cleavage of the peroxide (O–O) bond during fragmentation. Note that the sodium ion is centered in and slightly above the binding cavity and is equidistant from the oxygen atoms of TATP (Fig. 3c), probably due to steric effects related to the size of the Na+ ion and the cavity.14
The results show that DESI can be used as a screening method for the direct detection of trace amounts of TATP in-situ from a wide variety of surfaces (paper, brick, and metal) and that it is applicable to the detection of TATP present in various complex matrices. The method is fast (< 5 seconds to obtain spectra, no sample preparation), highly selective and gives low nanogram detection limits in all of the cases studied. Addition of alkali metals to the spray solution represents an easy way to improve the selectivity and sensitivity for TATP detection by complexation reactions during DESI analysis. The unique CID behavior of TATP complexes with alkali metals, and the remarkable retention of non-covalently-bound alkali metal ions in TATP during CID, is a fingerprint for this peroxide explosive.
This work was funded by the Office of Naval Research and the National Science Foundation (CHE 041278). We thank Professor Samuel Hernández, Department of Chemistry, University of Puerto Rico-Mayagüez, for the TATP samples.
Notes and references
- G. J. McKay, Kayaku Gakkaishi, 2002, 63, 323–329 Search PubMed.
- G. M. White, J. Forensic Sci., 1992, 37, 652–656.
- H. K. Evans, F. A. J. Tulleners, B. L. Sanchez and C. A. Rasmussen, J. Forensic Sci., 1986, 31, 1119–1125 CAS.
- L. R. Ember, Chem. Eng. News, 2005, 83, 11.
- J. C. Oxley, J. L. Smith and H. Chen, Propellants, Explos., Pyrotech., 2002, 27, 209–216 CrossRef CAS.
- J. C. Oxley, J. L. Smith, K. Shinde and J. Moran, Propellants, Explos., Pyrotech., 2005, 30, 127–130 CrossRef CAS.
- R. Schulte-Ladbeck, P. Kolla and U. Karst, Anal. Chem., 2003, 75, 731–735 CrossRef CAS.
- S. Zitrin, S. Kraus and B. Glattstein, Proc. Int. Symp. Anal. Detect. Explos., Quantico, VA, 1983, Fed. Bur. Invest., Washington, DC, 1983, pp. 137–141 Search PubMed.
- R. Wolffenstein, Chem. Ber., 1895, 28, 2265–2269.
- B. T. Fedoroff, Encyclopedia of Explosives and Related Items, Vol. 1, Picatinny Arsenal, Dover, NJ, 1960, pp. A42–A45 Search PubMed.
- J. Yinon, Forensic and environmental detection of explosives, John Wiley, Chichester, NY, 1999, pp. 10–11 Search PubMed.
- R. Meyer, J. Köhler and A. Homburg, Explosives, 5th edn., John Wiley-VCH, Weinheim, 2002, p. 346 Search PubMed.
- A. J. Bellamy, J. Forensic Sci., 1999, 44, 603–608 CAS.
- F. Dubnikova, R. Kosloff, Y. Zeiri and Z. Karpas, J. Phys. Chem. A, 2002, 106, 4951–4956 CrossRef CAS.
- L. Widmer, S. Watson, K. Schlatter and A. Crowson, Analyst, 2002, 127, 1627–1632 RSC.
- WO Pat., 9943846 A1, 1999. US Pat., 6 767 717 B1, 2004 Search PubMed.
- Z. Takats, J. M. Wiseman, B. Gologan and R. G. Cooks, Science, 2004, 306, 471–473 CrossRef CAS.
- Z. Takats, I. Cotte-Rodriguez, N. Talaty, H. W. Chen and R. G. Cooks, Chem. Commun., 2005, 1950–1952 RSC.
- I. Cotte-Rodriguez, Z. Takáts, N. Talaty, H. Chen and R. G. Cooks, Anal. Chem., 2005, 77, 6755–6764 CrossRef CAS.
- N. A. Milas and A. Golubovic, J. Am. Chem. Soc., 1959, 81, 6461–6462 CrossRef CAS.
- Z. Takats, J. M. Wiseman, B. Gologan and R. G. Cooks, Anal. Chem., 2004, 76, 4050–4058 CrossRef CAS.
- R. Schulte-Ladbeck and U. Karst, Anal. Chim. Acta, 2003, 482, 183–188 CrossRef CAS.
- D. Muller, A. Levy, R. Shelef, S. Abramovich-Bar, D. Sonenfeld and T. Tamiri, J. Forensic Sci., 2004, 49, 935–938 CAS.
- X. M. Xu, A. M. van de Craats, E. M. Kok and P. C. A. M. de Bruyn, J. Forensic Sci., 2004, 49, 1230–1236 CAS.
- F. Dubnikova, R. Kosloff, J. Almog, Y. Zeiri, R. Boese, H. Itzhaky, A. Alt and E. Keinan, J. Am. Chem. Soc., 2005, 127, 1146–1159 CrossRef CAS.
- R. R. Julian, J. A. May, B. M. Stoltz and J. L. Beauchamp, J. Am. Chem. Soc., 2003, 125, 4478–4486 CrossRef CAS.
- R. R. Julian, J. A. May, B. M. Stoltz and J. L. Beauchamp, Angew. Chem., Int. Ed., 2003, 42, 1012–1015 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional data. See DOI: 10.1039/b515122h |
| This journal is © The Royal Society of Chemistry 2006 |
