Naked-eye detection of fluoride ion in water: a remarkably selective easy-to-prepare test paper†
Zhi-hua Lin, Sheng-ju Ou, Chun-ying Duan*, Bing-guang Zhang and Zhi-ping Bai*
Coordination Chemistry Institute, State Key Laboratory of Coordination Chemistry, Nanjing 210093, P. R. China. E-mail: duancy@nju.edu.cn
First published on 9th January 2006
Abstract
A test paper for high-selectivity detecting fluoride ion in natural aqueous environments without any spectroscopic instrumentation was achieved by using Ru-bipy based quinonehydrazone as a chromo- and fluorogenic hybrid chemosensor.
The design of an artificial receptor capable of selective interactions with simple water-soluble anions remains the basis of many important subdisciplines of supramolecular chemistry, which investigate a variety of functions, including recognition, sensing, catalysis, etc.1,2 In particular, the sensing of fluoride ion has attracted growing attention,3 because of its great potential for biological and industrial applications,4 and fluoride’s unique properties compared to its congeners as a result of relative size and electronegativity. While a number of compounds that are able to bind fluoride ions with high affinity and selectivity have been reported,5 the challenges of detecting and amplifying the fluoride ion binding event to produce a measurable output still remain. Of particular interest in this regard are colorimetric anion sensors, species that would allow the naked-eye detection of fluoride without resorting to any spectroscopic instrumentation.6 Here, we report a remarkable selective Ru-based fluoride chromo- and fluorogenic hybrid chemosensor 1 of a Schiff-base compound HL, 1,10-phenanthroline-5,6-dione 2,4-dinitrophenylhydrazone.7 It contains a quinonehydrazone group that can be transformed to azophenol form in the presence of fluoride ion (Scheme 1),8 from which significant changes in absorption spectra and color of the solution can be expected. Furthermore, the existence of a photoactive Ru(bipy)3 moiety (bipy = 2,2′-bipyridine) not only enhances the affinity through electrostatic interactions, but also provides fluorogenic as well as chromogenic sensing.9
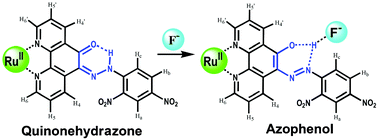 | ||
| Scheme 1 Proposed mode of anion binding of 1. | ||
Compound 1 was prepared by reaction of 2,4-dinitrophenylhydrazine with bis(2,2′-bipyridine)(1,10-phenanthroline-5,6-dione) RuII in the presence of H3PO4 and crystallized as PF6−salt.‡ Elemental analyses and spectroscopic measurements suggested the formation of [Ru(bipy)2(HL)](PF6)2. ESI-MS of the complex 1 in methanol solution showed two strong peaks at m/z = 803.1 and 948.9 (Fig. S1)†, which were assigned to [Ru(bipy)2L]+ and [Ru(bipy)2(HL)(PF6)]+, respectively, indicating that complex 1 was stable in solution. The UV-vis spectrum exhibited a strong absorption band at about 475 nm with a shoulder at about 450 nm, which was assigned to the 2,4-dinitrophenylhydrazone-centered charge transfer (CT) and the metal-to-ligands charge transfer (MLCT) of the Ru(bipy)2 moiety,9 respectively. Addition of F− to the solution of 1 caused a dramatic change in color from orange to blue-violet, which was accompanied by a new intense absorption band centered at about 580 nm in the UV-vis spectrum. We ascribed this new band to the ligand(HL)-based charge transfer (CT) of the azophenol tautomer, which was induced by an incipient proton-transfer from the quinonehydrazone tautomer to fluoride ion.8 The presence of two sharp isosbestic points at 390 nm and 500 nm indicated that only 1 and 1·F− adducts coexisted. The titration profile at 580 nm (insert in Fig. 1) supported the formation of 1∶ 1 stoichiometry adduct with an association constant logK being 6.23 ± 0.03 (calculated through nonlinear least-squares fitting).
 | ||
| Fig. 1 UV-vis titration of 1 in MeCN (2.5 × 10−5 M) solution upon addition of fluoride ion as TBA salt. Inset: absorbance at 580 nm vs. number (n) of mole equivalents of F− ion added. | ||
The sensing of 1 for halide anions such as Cl−, Br−, I−, and more complicated anions such as HSO4−, NO3− H2PO4− (all as tetra-n-butylammonium salts, TBA salts) were also studied through UV-vis spectra in MeCN solutions (Fig. S2).† The photograph in Fig. 2 showed the color changes after addition of equimolar amounts of these anions to the MeCN solution of 1. The color changes occurred only when fluoride anion was added, other anions failed to cause any significant color change.
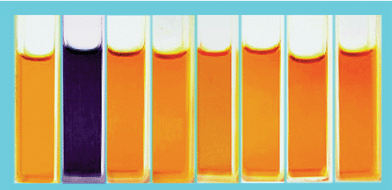 | ||
| Fig. 2 Color changes observed for 1 in MeCN solution (5 × 10−5 M) upon addition of one mole equivalent of anions as TBA salts: From left to right: free 1, F−, Cl−, Br−, I−, HSO4−, NO3− and H2PO4−. | ||
Furthermore, the presence of F− also induced significant enhancement of the luminescence intensities of Ru-based receptor 1 at 630 nm by exciting the CH3CN solution of 1 at 465 nm (Fig. 3). The titration curve of F− was consistent with a proposed 1 ∶ 1 host–guest binding stoichiometry with the association constant logK being 6.43 ± 0.05. Upon addition of anions such as Cl−, Br−, I−, HSO4− and NO3−, no detectable changes were observed. Considering the high selectivity for F− over other anions and the convenience without resorting to any spectrometer, the compound 1 provided a great advantage for detecting fluoride ion. It is suggested that the high selectivity might be attributed to the strong intramolecular N-H⋯O hydrogen bonding of 1, from which the hydrogen atom was fastened, and only the anion showing the most electronegative property had the potential to form additional hydrogen bonding.11
 | ||
| Fig. 3 Luminescence spectra of 1 in MeCN solution (2.5 × 10−5 M) after the addition of 1 equivalent of representative anions (top). Luminescence spectra of 1 in MeCN solution (2.5 × 10−5 M) and in the presence of F− with different concentration (bottom). | ||
As a validation of the above verdict, the binding property of the receptor 1 was studied by 1H NMR spectroscopy (Fig. 4). The addition of F− into an acetonitrile-d3 solution of 1 resulted in significant perturbations of the most notably NH signal downfield shift from 9.11 to 9.87 ppm, and exhibited a doublet as a result of coupling with the fluoride (J = 30 Hz), and the similar 1H–F coupling has been reported in a cryptand fluoride receptor.10 This result demonstrated the potential hydrogen bonding including the fluoride anion. The signals of Ha and Hb exhibited progressive upfield shifts, whereas the signal of Hc exhibited significant downfield shift. The promotion of the downfield suggested that the complexed fluoride anion positioned near the Hc proton.11 The evidence above was consistent with the formation of a 1·F− H-bonding host–guest complex. To further investigate the potential anion–receptor complexation, UV/vis–pH titrations upon addition of F− and OH− of the peak at about 580 nm were displayed (Fig. S3).† As can be expected, the presence of F− and OH− caused different titration curves from pH 6.0 to 12.0. Such a result supported the formation of a 1·F− H-bonding host–guest complex.
 | ||
| Fig. 4 1H NMR spectra of 1 in acetonitrile-d3 (5 × 10−2 M) with the presence of 2 equivalent molar ratio of fluoride anion. | ||
Generally, receptors for anions based solely on hydrogen-bonding interactions cannot serve as efficient sensors in aqueous media, due to the strong solvent competition. To avoid the competing solvation effect of water, we prepared a test paper of 1 for inspecting F− in aqueous environments by putting a filter paper (3 × 0.5 cm2) into the acetonitrile solution of 1 (2.0 ×10−3 M) and then drying it in the air. For detecting the fluoride anion in water, a test paper was immersed in the test aqueous fluoride-containing solution for several seconds then dried in the air. Fig. 5 exhibits the color changes of the test papers with different fluoride concentrations at pH ≈ 7. Clearly, the test paper can detect F− in aqueous solution at a low limit about 10 ppm (10 mg L−1). Other anions such as Cl−, Br−, I−, HSO4− and NO3− did not cause any detectable changes. In fact, sensing F− in natural aqueous environments without any spectroscopic instrumentation has been very useful in preventing fluorosis caused by the fluoride toxicity12 in undeveloped regions. However, since many other anions such as chloride were present in most water at much higher concentrations than fluoride, it was important to know that chloride (or other common anions) did not cause color changes at concentrations normally found in water. As a complete study of the real application of fluoride detection, test papers were immersed in aqueous solutions having different fluoride concentrations in the presence of 10 g L−1 chloride anion (or/and other anions listed above). Similar color changes to those of solutions containing only fluoride showed the test papers provided the practical means to inspect fluoride anion concentrations in the wilderness.
 | ||
| Fig. 5 The color changes of the test papers for detecting fluoride ion in neutral aqueous solution with different F− concentrations. | ||
In summary, we have presented here a rational strategy for the development of a new highly selective chromo-sensor to detect F− in natural aqueous environments without any spectroscopic instrumentation. The proton transfer from the quinonehydrazone tautomer to fluoride anion induced the formation of azophenol tautomer and caused a dramatic change in color from orange to blue-violet. The easy-to-prepare fluoride test paper can detect F− in aqueous solution at a low limit about 10 ppm (10 mg L−1). This cheap sensing probe would be advantageous in the prevention of fluorosis in undeveloped regions.
This work was supported by the National Natural Science Foundation of China and the Eduction Ministry of China.
Notes and references
- J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspective. VCH, Weinheim, 1995 Search PubMed
; J. W. Steed and J. L. Atwood, Supramolecular Chemistry: A Concise Introduction. Wiley, Chichester, 2000 Search PubMed
.
- For recent reviews on anion receptors, see: R. Martínez-Máñez and F. Sancenón, Chem. Rev., 2003, 103, 4419–4467 Search PubMed
; P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486–516 CrossRef CAS
; S. L. Wiskur, H. Ait-Haddou, E. V. Anslyn and J. J. Lavigne, Acc. Chem. Res., 2001, 34, 963–972 CrossRef
.
- T. H. Kim and T. M. Swager, Angew. Chem., Int. Ed., 2003, 42, 4803–4806 CrossRef
; Y. Kubo, M. Yamamoto, M. Ikeda, M. Takeuchi, S. Shinkai, S. Yamaguchi and K. Tamao, Angew. Chem., Int. Ed., 2003, 42, 2036–2040 CrossRef CAS
; A. R. Bassindale, M. Pourny, P. G. Taylor, M. B. Hursthouse and M. E. Light, Angew. Chem., Int. Ed., 2003, 42, 3488–3490 CrossRef CAS
.
- C. R. Cooper, N. Spencer and T. D. James, Chem. Commun., 1998, 1365–1365 RSC
; C. B. Black, B. Andrioletti, A. C. Try, C. Ruiperez and J. L. Sessler, J. Am. Chem. Soc., 1999, 121, 10438–10439 CrossRef CAS
.
- C. A. Ilioudis, D. A. Tocher and J. W. Steed, J. Am. Chem. Soc., 2004, 126, 12395–12400 CrossRef CAS
; B. G. Zhang, P. Cai, C. Y. Duan, R. Miao, L. G. Zhu, T. Niitsu and H. Inoue, Chem. Commun., 2004, 2206–2207 RSC
; M. L. Lehaire, R. Scopelliti, H. Piotrowski and K. Severin, Angew. Chem., Int. Ed., 2002, 41, 1419–1422 CrossRef CAS
.
- E. J. Cho, J. W. Moon, S. W. Ko, J. Y. Lee, S. K. Kim, J. Yoon and K. C. Nam, J. Am. Chem. Soc., 2003, 125, 12376–12377 CrossRef CAS
; T. Mizuno, W. H. Wei, L. R. Eller and J. L. Sessler, J. Am. Chem. Soc., 2002, 124, 1134–1135 CrossRef CAS
; L. Zhou, X. Zhang and S. K. Wu, Chem. Lett., 2004, 33, 850–851 CrossRef CAS
; J.-L. Fillaut, J. Andriès, L. Toupet and J.-P. Desvergne, Chem. Commun., 2005, 2924–2926 RSC
.
- G. F. Smith and F. W. Cagle, J. Org. Chem., 1947, 12, 781–784 CrossRef CAS
.
- B. Chiswell, F. Lions and M. L. Tomlinson, Inorg. Chem., 1964, 3, 492–499 CrossRef CAS
; H. Tong, G. Zhou, L. Wang, X. Jing, F. Wang and J. Zhang, Tetrahedron Lett., 2003, 44, 131–134 CrossRef CAS
.
- P. D. Beer, F. Szemes, V. Balzani, C. M. Salà, M. G. B. Drew, S. W. Dent and M. Maestri, J. Am. Chem. Soc., 1997, 119, 11864–11875 CrossRef CAS
; P. Anzenbacher, Jr., D. S. Tyson, K. Jursíková and F. N. Castellano, J. Am. Chem. Soc., 2002, 124, 6232–6233 CrossRef
.
- M. Boiocchi, L. D. Boca, D. E. Gomez, L. Fabbrizzi, M. Licchelli and E. Monzani, J. Am. Chem. Soc., 2004, 126, 16507–16514 CrossRef CAS
; A. B. Descalzo, K. Rurack, H. Weisshoff, R. Martínez-Máñez, M. D. Marcos, P. Amorós, K. Hoffmann and J. Soto, J. Am. Chem. Soc., 2005, 127, 184–200 CrossRef CAS
.
- S. K. Kang, J. M. Llinares, D. Powell, D. VanerVelde and K. Bowman-James, J. Am. Chem. Soc., 2003, 125, 10152–10153 CrossRef CAS
; M. Shionoya, H. Furuta, V. Lynch, A. Harriman and J. L. Sessler, J. Am. Chem. Soc., 1992, 114, 5714–5722 CrossRef CAS
.
- M. Diesendorf, J. Colquhoun, B. J. Spittle, D. N. Everingham and F. W. Clutterbuck, Aust. N. Z. J. Public Health, 1997, 41, 29–44 Search PubMed
; J. Nomura, H. Imai and T. Miyake, in Emerging Technologies In Hazardous Waste Management, ed. D. W. Teder, F. G. Pohlanf, ACS Symp. Ser., No. 422, American Chemical Society, Washington, DC, 1990 Search PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: ESI-MS spectra (Fig. S1), UV-vis spectra in the presence of anions (Fig. S2) and UV/vis–pH titrations upon addition of F− and OH− (Fig. S3) of compound 1. See DOI: 10.1039/b514337c |
| ‡ A solution of 2,4-dinitrophenylhydrazine (0.198 g, 1 mmol) and bis(2,2′-bipyridine)(1,10-phenanthroline-5,6-dione)rutheniumbis (hexafluorophosphate) (0.9 g, 1 mmol) in mixed solvent comprised of 5 mL H3PO4, 25 mL MeCN and 25 mL EtOH was refluxed for 8 h under N2. The solution was concentrated to 5 mL. An orange precipitate formed by adding the solution to a saturated aqueous solution of KPF6 was collected and dried under vacuum. Yield 60%. Anal. calc. for (C10H8N2)2Ru(C18H10N6O5)(PF6)2: C, 41.7 H, 2.4 N, 12.8%. Found: C, 41.6 H, 2.5 N, 12.7%. 1H NMR (500 MHz, CD3CN), δ (ppm): 9.11 (br, 1H, −NH), 8.97 (br, 1H), 8.74 (d, 1H), 8.52 (m, 6H), 8.05 (m, 7H), 7.68 (m, 4H), 7.58 (t, 1H), 7.53 (t, 1H), 7.37 (m, 2H), 7.32 (m, 2H). |
| This journal is © The Royal Society of Chemistry 2006 |
