Fabrication of monodisperse colloidal array with confinement effects
Yuan
Jiang
a,
Xun
Li
a,
Hongjiang
Liu
a,
Zheng
Xu
*a,
Xiaoping
Shen
a,
Xiang
Ma
b and
Ziling
Xue
c
aState Key Laboratory of Coordination Chemistry and Laboratory of Solid State Microstructure, Nanjing University, China. E-mail: zhengxu@netra.nju.edu.cn; Fax: 86-25-83314502; Tel: 86-25-83595459
bCenter of Material Analysis, Nanjing University, China
cDepartment of Chemistry, University of Tennessee, Knoxville, TN 37996-1600, USA
First published on 14th November 2005
Abstract
A monodisperse 1D colloidal array is prepared from monomer directly combining precipitation polymerization and confinement effects.
Colloidal arrays have many useful functions as constituent materials, removable templates and diffractive elements.1 Commonly, fabrication of a colloidal array needs two steps: preparation of monodisperse spherical colloids2 and assembly of the colloids into an ordered array.3 As far as we know, there has been no one-step method for assembling colloids into the special confined space from monomers directly till now. Since in biomineralization, biomaterials are always formed and assembled concomitantly, it would be valuable to develop such a one-step method for organizing microstructures based on the coupled processes in which formation of colloids and their assembly occur concomitantly. However, in contrast to colloidal inorganic nanoparticles, which could be synthesized and self-assembled in a coupled process because of strong attractive interactions between them first reported by Li et al.,4 monodisperse colloids (here meaning those usually bigger than 50 nm) lack the inherent materials-building function, thus other confining conditions should also be included.
Constrained and organized media is another research focus to research polymerization as well as polymer self-assembly. In polymerization, micelles, lipid bilayers, liquid crystals, organic crystals, as well as micro- and meso-porous materials have been used as constrained media and products always show several features such as monodisperse molecular weight (Mw), high Mw or higher-ordered structures than those formed in non-constrained and bulk media.5 For example, the Martin group has found that polyaniline (PAN) prepared in alumina oxide (AAO) channels shows better conductivity than that in bulk solution.5 As to self-assembling, research mainly focuses on polymer, especially copolymer self-assembly.6 The Russell group recently discovered that when the pore diameter of the AAO membrane was small and incommensurate with the copolymer period, the imposed curvature produced unusual copolymer morphologies.6 Also, triblock copolymers such as P123 and F127 can be used as templates to prepare inorganic meso-structures in AAO membranes.7
Our goal is one-step preparation of a colloidal array with an AAO membrane as confining environment, combining polymer chains formation, chains self-assembling into monodisperse colloids and colloids assembling together to form a colloidal array within channels (Scheme 1). Monodisperse spherical colloids were formed along the channels with proper solvent polarity, or other colloidal morphologies such as rods, wires and particles were formed. Further, the colloidal array shows a blue-shift in PL spectra, which proves the existence of confinement effects.
 | ||
| Scheme 1 Fabrication steps and sample morphologies. | ||
N-vinylcarbazole (NVK, A.R.) was used after recrystallization in absolute methanol (A.R.) twice and azobisisobutyronitrile (AIBN, C.R.) was used after recrystallization in acetone (A.R.). Pyridine (A.R.) was used after distillation. Poly(N-vinylcarbazole) was bought from Aldrich Chemical Co (PVK, Mw = 80,000). KOH (C.R.) was used directly. The AAO membranes were purchased from Whatman Corp. and the pore diameters determined by SEM were about 200–250 nm.
In a typical example, 0.3 g NVK, 20 mg AIBN and a given amount of pyridine/water (abbreviated as p/w, listed in Table 1) as monomer, initiator and dispersion media respectively were added into a three-necked flask under ultrasound for 1 minute to form a homogeneous solution. A piece of AAO membrane was put in the above solution. After bubbling nitrogen for 15 minutes, the flask was sealed and placed in the dark at −10 °C for 24 hours to fill the channels with solution, which is the key step to form a high-density polymer colloidal array in the membrane. Then the flask was heated to 65 °C ± 1 °C and reacted for 12 hours. The AAO membrane turned opaque and then was removed from the solution.
Samples were measured with SEM, photoluminescence (PL) and time resolved fluorescence spectrometry respectively. In the preparation of SEM (Hitachi X650/EDAX, PV9100) samples, after carefully removing the polymer on the surface of the membranes with sandpaper, the membranes were cleaved and etched in 10% KOH aqueous solution for 2 minutes and then glued to the tack and sputtered with gold. For PL and time resolved fluorescence spectra (SLM 48000Dscf/AB2 spectrofluorometer, equipped with a HeCd laser with λ = 325 nm), the membranes after sanding on the surface were mounted on the sample holder and the laser was perpendicular to the plane of the AAO membrane.
Fig. 1A–1C exhibit the side and top views of the SEM images of the sample a respectively. Fig. 1A shows that monodisperse colloids form highly ordered 1D arrays along channels and the size of the colloids is quite uniform. Fig. 1B shows the ordered structure in Fig. 1A with higher magnification. Part of the array structure was destroyed because of cleaving membranes and etching in the KOH aqueous solution. Fig. 1C shows a top view of the array composed of ordered monodisperse colloids about 200 nm in diameter on top of the membranes. Fig. 1D shows that colloids in sample a′ (sample a means colloids formed in channels and sample a′ means those formed out of membranes) are multidisperse and their diameter ranges from 0.5 µm–1.2 µm. In short, colloids in sample a are monodisperse and smaller in diameter while those in sample a′ are multidisperse and bigger. Those multidisperse colloids in sample a′ can be easily explained because in precipitation polymerization, monodisperse colloids are formed only under special conditions such as in near θ solvent or with cross-linkers.2 As for sample a, the confinement effects should be in favor of nucleation simultaneously, which is the prerequisite for formation of monodisperse colloids and colloidal arrays in channels.
 | ||
| Fig. 1 SEM images of side view (A), (B) and top view (C) of 1D array of sample a. (D) is PVK colloids formed in bulk solution of sample a. (E) is the side view of sample b and (F) is the top view of sample c. (G) shows the side view of sample d. (H) is the side view of sample e. | ||
Then we consider the relationship of solvent polarity with the colloidal morphology in channels (Table 1). As shown in Fig. 1E, colloidal rods are formed when p/w = 3/1 (sample b). The length of the rods is from 0.4 µm–1 µm. The rods are formed because when the colloids are bigger than the channel diameter, they choose to grow further along the channels. As the polarity is further decreased to p/w = 4/1 (sample c), ordered arrays of colloidal wires (a wire is longer than a rod) are generated, which is clearly shown in Fig. 1F. Conversely, increasing the polarity of the solvent to p/w = 2/1 (sample d) causes the solubilities of NVK and AIBN to become smaller. Thus only a few smaller and multidisperse colloidal particles from 100–200 nm are formed in the channels (Fig. 1G). Thus, the polarity and solvency of the dispersion media are important parameters for the size monodispersity and shape of colloids, because they have a crucial effect on the rate of the nucleation and the number of nuclei.
Additional to the dispersion medium with p/w = 9/4, pure methanol is an alternative suitable dispersion medium for constructing colloidal arrays in membranes. Fig. 1H shows the side view of the SEM image of the sample e prepared in pure methanol.
PL spectra of samples a, b, c, a′ and commercial PVK powders are shown in Fig. 2A. (The particles in sample d are so few that no PL signals were detected.) Samples a, b and c show that the fluorescence emission peak appears at 413 nm, which is 7 nm blue-shifted from that of sample a′ and commercial PVK powders (curve x) at 420 nm. It has been well recognized that the emission at 420 nm results from intramolecular triplet–triplet annihilation, so called delayed fluorescence, and the emitting species have a configuration in which the interaction between neighboring chromophoric groups is strong enough.8 Due to the confinement effects in the channels, PVK chains in colloids may be packed more compactly, which causes neighboring chromophoric groups to be closer to each other and results in the existence of stronger interactions, therefore leading to a 7 nm blue-shift from 420 nm. Further, from time resolved fluorescence spectra, the lifetime of three samples is about 7 ns, while the lifetime of sample a′ is 14 ns and that of the commercial powder is 15 ns, which may also prove the existence of confinement effects.
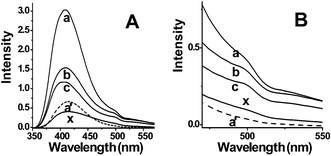 | ||
| Fig. 2 Fig. 2A is PL spectra of samples a, b, c, a′ and commercial powder (curve x). Fig. 2B is part of magnified Fig. 2A. Spectra of samples a, b and c are on the same absolute scale and emission from the AAO membrane has been abstracted. Signals in sample d are too weak to detect. | ||
From Fig. 2B, there is emission at about 505 nm for samples a, b and c, but no such emission in sample a′ and the commercial powder (curve x). It was reported that there is no phosphorescence emission at about 505 nm when the Mw of PVK is larger than 106.8 Based on the previous work and the PL spectra, we may infer that the Mw of colloids in channels is less than those out of channels. Combined with the information that the diameter of sample a is smaller than that of sample a′, we may conclude that colloids in channels are not grown fully. This is reasonable because when the diameter of the colloids grows near that of the channels, monomers cannot be transported further from bulk solutions. Thus the colloids in channels stop growing while those out of channels continue growing, which causes the diameter and Mw of the colloids in sample a to be less than those in sample a′.
This is the first report that a colloidal array was constituted in channels, combining precipitation polymerization and confinement effects together. Compared to previous reports about polymerization within constrained media, we firstly introduced heterogeneous polymerization and the results are somewhat different from previous reports. The structure of polymer chains formed with confinement effects is different from that formed in bulk solution, which is proved by PL spectra. The narrow Mw dispersion within channels can be detected since narrow colloids diameter distribution always corresponds to narrow Mw dispersion. However, in contrast to previous reports, the Mw of colloids within channels is lower than that in bulk solution, which is attributed to a shortage of monomer when the colloids are so big that they block the channels and no further monomer can be supplied. It is a great advantage to understand the confinement effects on the polymerization and therefore, to construct colloidal arrays in the desired complex environments.
We thank the National Natural Science Foundation of China for financial support under major project 20490210 and project 20371026.
Notes and references
- Y. N. Xia, B. Gates, Y. D. Yin and Y. Lu, Adv. Mater., 2000, 12, 693 CrossRef CAS.
- W. Stöber and A. Fink, J. Colloid Interface Sci., 1968, 26, 62 CrossRef; K. Li and H. D. H. Stöver, J. Polym. Sci., Part A: Polym. Chem., 1993, 31, 3257 CAS.
- A. Van Blaaaderen, R. Ruel and P. Wiltzius, Nature, 1997, 385, 321 CrossRef CAS; P. Jiang, J. F. Bertone, K. S. Hwang and V. L. Colvin, Chem. Mater., 1999, 11, 2132 CrossRef CAS; S. H. Park, D. Qin and Y. X. Xia, Adv. Mater., 1998, 10, 1028 CrossRef CAS.
- M. Li, H. Schnablegger and S. Mann, Nature, 1999, 402, 393 CrossRef CAS.
- K. Tajama and T. Aida, Chem. Commun., 2000, 2399 RSC; K. Ito, K. Tanaka, H. Tanaka, G. Imai, S. Kawaguchi and S. Itsuno, Macromolecules, 1991, 24, 2348 CrossRef CAS; J. H. Fendler, Science, 1984, 223, 888 CrossRef CAS; S. I. Stupp, S. Son, H. C. Lin and L. S. Li, Science, 1993, 259, 59 CrossRef CAS; A. Matsumoto, T. Odani, K. Sada, M. Miyata and K. Tashiro, Nature, 2000, 405, 328 CrossRef CAS; C. G. Wu and T. Bein, Science, 1994, 6, 1109 CAS; K. Kageyama, J.-I. Tamazawa and T. Aida, Science, 1999, 285, 2113 CrossRef CAS; C. R. Martin, Science, 1994, 266, 1961 CrossRef CAS.
- K. Shin, H. Q. Xiang, S. I. Moon, T. Kim, T. J. McCarthy and T. P. Russell, Science, 2004, 306, 76 CrossRef CAS; M. Steinhart, J. H. Wendorff, A. Greiner, R. B. Wehrspohn, K. Nielsch, J. Schilling, J. Choi and U. Gösele, Science, 2002, 296, 1997 CrossRef CAS.
- G. Kickelbick, Small, 2005, 1, 168 Search PubMed.
- R. D. Burkhart, Macromolecules, 1983, 16, 820 CrossRef CAS; W. Klöpffer, D. Fischer and G. Naundorf, Macromolecules, 1977, 10, 450 CrossRef.
| This journal is © The Royal Society of Chemistry 2006 |
