Intein-mediated in vitro synthesis of lipidated Ras proteins†
D.
Gottlieb
ab,
C.
Grunwald
c,
C.
Nowak
c,
J.
Kuhlmann
*c and
H.
Waldmann
*ab
aMax Planck Institute of Molecular Physiology, Otto-Hahn-Str. 11, 44227, Dortmund, Germany. E-mail: herbert.waldmann@mpi-dortmund.mpg.de; Fax: (+49)231 133 2499; Tel: (+49) 133 2400
bDepartment of Chemical Biology, University of Dortmund, Otto-Hahn-Str. 6, 44227, Dortmund, Germany. E-mail: herbert.waldmann@mpi-dortmund.mpg.de; Fax: (+49)231 133 2499; Tel: (+49) 133 2400
cMax Planck Institute of Molecular Physiology, Department of Structural Biology, Otto-Hahn-Str. 11, 44227, Dortmund, Germany. E-mail: juergen.kuhlmann@mpi-dortmund.mpg.de; Fax: (+49) 133 2199; Tel: (+49) 133 2104
First published on 28th November 2005
Abstract
Fully functional lipid-modified Ras proteins suitable for the study of Ras–membrane interactions and embodying exclusively native amide bonds can be synthesized in preparative amounts by means of Expressed Protein Ligation.
The superfamily of the Ras-type GTPases includes more than 100 lipidated proteins which are key regulators of numerous important processes like cell growth, differentiation, and apoptosis (Ras), organization of the cytoskeleton (Rho) or vesicular transport (Rab).1 In order to fulfil their biological functions these proteins need to be modified with farnesyl or geranylgeranyl thioethers or palmitic acid thioesters at their C-termini. For the study of their function, properties and structure semisynthetic proteins composed of the native protein core obtained via molecular biology techniques and modified C-termini obtained by means of organic synthesis and embodying native or non-natural lipid groups alone or together with further tags, labels and reporter groups have proven to be invaluable tools.2–4 Ideally such synthetic proteins should incorporate exclusively native peptide bonds and no non-native structures resulting from coupling of the protein core to the synthetic C-terminus. Currently the most powerful techniques for native protein synthesis are native chemical ligation (NCL)5 and expressed protein ligation (EPL).6 In both techniques the thioester of a large peptide unit (synthetic or obtained via an engineered expression system) is ligated to a (modified) peptide carrying an unprotected cysteine at the N-terminus.
To date only Rab proteins have become amenable to synthesis by means of EPL. This Rab-synthesis required optimization of the ligation conditions and finally became feasible because in the case of the Rabs the Rab escorting protein REP-1 could be used for stabilization and isolation of the coupling products.2,3 While the availability of such a chaperone at first glance may seem to be of secondary importance, in fact it proved to be the decisive feature of Rab synthesis by means of EPL. For the Ras proteins and other Ras-type GTPases such a crucial helper protein is not available. Therefore, the methodology developed for the Rab GTPases does not provide a general solution for the synthesis of lipid-modified proteins. Despite intensive attempts to synthesize fully functional lipid-modified Ras proteins with exclusively native amide bonds to date a successful synthesis of these protein conjugates has not been achieved.
Here we report on the first synthesis of two Ras-type proteins by means of expressed protein ligation.
For the synthesis of the protein core the DNA sequence coding for truncated H-Ras (amino residues 1–180) was cloned into the IMPACT (Intein-Mediated Purification with an Affinity Chitin-binding Tag) vector pTYB2 (New England Biolabs).7 The fusion protein was expressed in E. coli BL21 (DE3) and purified on a chitin affinity column. The Ras moiety was released from the column with 200 mM mercaptoethanesulfonic acid sodium salt (MESNA) resulting in the Ras-MESNA-thioester (see also the supporting information). Since the G-domains of all Ras isoforms are almost identical, we selected the structurally and biophysically well characterized H-Ras as a prototype for the implementation of EPL in the Ras field.
For coupling to the Ras thioester two different lipidated peptides were synthesized. Dodecapeptide 3 which represents the farnesylated C-terminus of the K-Ras 4B protein8 and octapeptide 4 which is characteristic for the geranylgeranylated C-terminus of the D-Ral protein9 were obtained by solid phase synthesis employing an Fmoc/Aloc strategy and a trityl linker developed earlier for the synthesis of polybasic lipidated peptides10 (Scheme 1). To this end, Fmoc/All-protected lysine was attached to the solid phase via the side-chain amino group (1). After selective removal of the allyl ester S-farnesylated or S-geranylgeranylated cysteine methyl ester was attached to the lysine to yield compounds 2a and 2b. Then the amino acid chain was elongated in the N-terminal direction employing amino acid building blocks with an Fmoc group at the N-terminus and Aloc groups attached to the side chains of the lysine and arginine residues. The terminal cysteine was introduced with the thiol masked as the tert-butyl disulfide. After N-terminal deprotection the Aloc groups were cleaved and the lipid-modified peptides were released from the polymeric carrier by treatment with 1% TFA in CH2Cl2. Analysis of the crude product revealed that the target peptide had been formed together with side products of lower molecular weight; their structures could not be assigned to deletion sequences or truncated peptides although they are expected to arise from incomplete coupling steps within the polybasic sequence. Diketopiperazine formation was not observed. Employing 10% acetic acid anhydride in pyridine for capping of unreacted free amino groups after coupling steps during the synthesis did not improve the purity of the crude products. After purification by HPLC the target peptides were obtained in ca. 20% overall yield (Scheme 1).
 | ||
| Scheme 1 Solid phase synthesis of the farnesylated C-terminus of K-Ras 4B 3 and the geranylgeranylated C-terminus of D-Ral 4 and their coupling to expressed Ras thioester. a) Pd(PPh3)4, phenylsilane, THF, argon; b) Fmoc-L-Cys(prenyl)-OMe, PyBOP, 4% NMM, DMF; c) i) Fmoc-L-AA, HATU, HOAt, DIEA, DMF; ii) piperidine, DMF; d) Pd(PPh3)4, piperidine, DMF, argon; e) TFA, TES, CH2Cl2; f) phosphate buffer, MESNA, argon. TFA = trifluoroacetic acid, PyBOP = benzotriazol-1-yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate, NMM = N-methylmorpholine, HATU = 2-(7-aza-1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate, HOAt = 1-hydroxy-7-azabenzotriazole, DIEA = N,N′-diisopropylethylamine, TES = triethylsilane. | ||
Ligation of the H-Ras(1–180)-MESNA thioester with the lipidated peptides was carried out in phosphate buffer (pH 7.5) under argon in the presence of 125 mM mercaptoethanesulfonic acid sodium salt and followed by means of SDS-PAGE gel electrophoresis (Fig. 1). As a result of the coupling the bands are clearly shifted to higher molecular weight. In the presence of ten equivalents of the peptide nearly quantitative conversion of the recombinant protein is achieved as indicated by SDS-PAGE gel electrophoresis. In the case of the D-Ral and the K-Ras peptide the coupling reaction was complete after 4 h. The proteins were characterized by LC-MS (H-Ras–K-Ras coupling product m/zobs = 21982.0, m/zcalc = 21981.1; H-Ras/D-Ral coupling product m/zobs = 21645.0, m/zcalc = 21645.7).
 | ||
| Fig. 1 Ligation of H-Ras-MESNA-thioester to K-Ras and D-Ral peptides 3 and 4. A: SDS-PAGE analysis of the chimeric H-Ras/K-Ras protein: H-Ras-MESNA-thioester (lane 1), H-Ras/K-Ras protein after purification (lanes 2–6); B: SDS-PAGE analysis of the chimeric H-Ras/D-Ral protein: H-Ras-MESNA-thioester (lane 1), crude ligation mixture after 4 h (lanes 2, 3), after 6 h (lanes 4, 5) and after 8 h (lanes 6, 7). M: molecular weight marker. | ||
Ligation reactions on a preparative scale resulted in up to 20% yield and gave milligram amounts of the desired protein after purification by ion exchange chromatography and gel filtration respectively (see the supporting information).
Activity of the semisynthetic H-Ras/K-Ras lipoprotein was proven in an interaction assay with the catalytic domain of the guanine-nucleotide-exchange factor (GEF) Sos11 that promotes the slow intrinsic nucleotide release of Ras by several orders of magnitude. Fig. 2 shows the exchange of protein-bound, fluorescently-labeled methylanthraniloyl-GDP (mant-GDP) by a 500-fold excess of non-labeled GDP after addition of Sos protein. For the native Ras proteins isoprenylation does enhance the catalytic activity of Sos significantly.12 In accordance with this finding the H/K-Ras coupling product displays the same change in properties when compared to the non-modified H-Ras(1–181)*mant-GDP control. Therefore both elements for Ras–Sos interaction are functional after native chemical ligation: the G-domain for nucleotide binding and catalytic interaction with Sos and the lipidated C-terminus that enhances the binding affinity of the GEF for Ras.
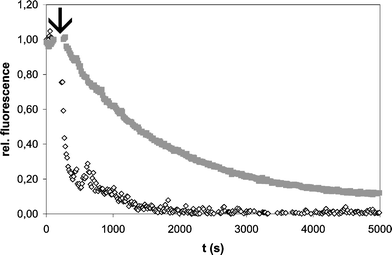 | ||
| Fig. 2 Sos stimulated nucleotide exchange. 200 nM of H/K-Ras lipoprotein (open diamonds) or H-Ras (AA 1–181) (grey squares), both complexed with mant (N-methylanthraniloyl)-GDP were incubated with 1 µM of human Sos1 protein (AA 564–1094) and GDP was added to a final concentration of 100 µM (arrow). The decay in fluorescence signal due to release of mant-GDP and consecutive replacement by GDP was monitored at 440 nm (excitation at 350 nm) in a Fluoromax II spectrometer (Horiba/Jobin-Yvon, Germany) at 20 °C. Buffer: 150 mM NaCl, 10 mM Hepes, pH 6.8. | ||
For H- and N-Ras a very recently identified deacylation/reacylation cycle steers the selective plasma membrane localization of these proteins.13 However, for the most important human Ras protooncogene K-Ras the underlying mechanisms for selective incorporation into the plasma membrane are less clear. Due to the polycationic nature of the K-Ras C-terminus, caused by a stretch of protonated basic amino acid side chains, passive diffusion of the protein to the negatively charged inner leaflet of the plasma membrane has been proposed as an alternative to an actively guided transport mechanism.14 In order to approximate this situation we generated surfaces mimicking differently charged membranes on biosensors for surface plasmon resonance (SPR) and analysed the binding of H-Ras/K-Ras chimera as a function of the surface composition. Such experiments have not been carried out before mainly due to insufficient access to preparative amounts of fully processed lipidated K-Ras protein. To this end, an L1-chip (BIAcore™) carrying a carboxymethylated dextran polymer with covalently attached lipophilic groups was loaded with small unilamellar vesicles (SUVs) composed of the neutral lipid palmitoyl-oleoyl-phosphatidyl choline (POPC) and different molar fractions of phosphatidyl serine (PS) as the negatively charged lipid (see the supporting information). The chip was then incubated with a solution of the semisynthetic protein in a Biacore1000™ instrument and adsorption and desorption of the protein were analysed via the SPR signal (Fig. 3).
 | ||
| Fig. 3 Insertion of H/K-Ras lipoprotein into a POPC/PS surface. POPC and POPC/PS membranes were generated by application of the corresponding vesicle suspension (0.5 mM) in buffer (150 mM NaCl, 10 mM Hepes, pH 6.8) to the surface of an L1 chip. Subsequently the H/K-Ras chimera was injected for 10 min at 20 °C followed by flushing the sensor surface with buffer for 30 min (dotted line, 15 min for 60% PS). X: proportion of POPC in the model membrane. | ||
The sensogram shows a rapid association of the protein to the model membranes that is significantly enhanced for all PS doped surfaces (20%, 40%, 60% PS respectively) compared to the pure POPC matrix. This observation indicates a clear contribution of the interaction between the protonated side chains in the C-terminus of the protein with negatively charged lipid in the model membrane. Therefore, the H/K-Ras chimera features the membrane binding properties expected for the natural, fully processed K-Ras lipoprotein.
Synthesis of Ras lipoproteins with a C-terminus descending from K-Ras 4B or D-Ral as described here is the second example for an EPL based strategy for the production of lipoproteins. To date successful intein ligation with a functional product is only established for geranylgeranylated Rab proteins and does require demanding conditions for coupling and purification including aggregation steps, washing with organic solvent, denaturation of the lipoprotein by guanidinium chloride and refolding in the presence of a chaperone.3 Due to the absence of an analogous partner to the Rab escorting protein 1 (REP-1) for Ras all attempts to adapt this procedure for EPL attachment of lipidated H- or N-Ras C-termini have resulted only in analytical amounts of product so far.15 The results presented here suggest that proper adjustment of the physical properties of the peptides for coupling, e.g. in the form of the polybasic K-Ras 4B and D-Ral lipopeptides is crucial for successful synthesis on the preparative scale.
In conclusion, we have demonstrated that Ras-type lipoproteins with native amide bonds can be synthesized in preparative amounts relevant for biochemical and biophysical studies by means of expressed protein ligation. Subsequent projects with K-Ras and Ral lipoproteins will address e.g. the detailed biophysical analysis of electrostatic modulation in K-Ras 4B plasma membrane binding.
These semisynthetic biopolymers combine the advantages of designed synthetic membrane anchors and large scale preparation of the protein core in bacteria.
The presence of the native linkage between both moieties makes it possible to further approximate the natural context of cellular signal transduction.
This research was supported by the Deutsche Forschungsgesellschaft, the Max Planck Gesellschaft and the Fonds der Chemischen Industrie.
Notes and references
- Reviews: (a) A. Wittinghofer and H. Waldmann, Angew. Chem., Int. Ed., 2000, 39, 4192 CrossRef CAS (and references therein); (b) A. Watzke, L. Brunsveld, T. Durek, K. Alexandrov, A. Rak, R. S. Goody and H. Waldmann, Org. Biomol. Chem., 2005, 3, 1157 RSC.
- L. Brunsveld, A. Watzke, T. Durek, K. Alexandrov, R. S. Goody and H. Waldmann, Chem. Eur. J., 2005, 11, 2756 CrossRef CAS.
- T. Durek, K. Alexandrov, R. S. Goody, A. Hildebrand, I. Heinemann and H. Waldmann, J. Am. Chem. Soc., 2004, 126, 16368 CrossRef CAS.
- R. Reents, M. Wagner, S. Schlummer, J. Kuhlmann and H. Waldmann, ChemBioChem, 2005, 6, 86 CrossRef CAS.
- P. E. Dawson and S. B. H. Kent, Annu. Rev. Biochem., 2000, 69, 923 CrossRef CAS.
- T. W. Muir, Annu. Rev. Biochem., 2003, 72, 249 CrossRef.
- S. Chong, Y. Shao, H. Paulus, J. Benner, F. B. Perler and M. Q. Xu, J. Biol. Chem., 1996, 271, 22159 CrossRef CAS.
- I. A. Prior and J. F. Hancock, J. Cell Sci., 2001, 114, 1603 Search PubMed.
- S. B. Cantor, T. Urano and L. A. Feig, Mol. Cell. Biol., 1995, 15, 4578 CAS.
- B. Ludolph, F. Eisele and H. Waldmann, ChemBioChem, 2002, 3, 901 CrossRef CAS.
- P. A. Boriack-Sjodin, S. M. Margarit, D. Bar-Sagi and J. Kuriyan, Nature, 1998, 394, 337 CrossRef CAS.
- P. McGeady, E. Porfiri and M. H. Gelb, Bioorg. Med. Chem. Lett., 1997, 7, 145 CrossRef CAS.
- O. Rocks, A. Peyker, M. Kahms, P. J. Verveer, C. Koerner, M. Lumbierres, J. Kuhlmann, H. Waldmann, A. Wittinghofer and P. I. H. Bastiaens, Science, 2005, 307, 1746 CrossRef CAS.
- J. R. Silvius, J. Membr. Biol., 2002, 190, 83 Search PubMed.
- V. Haridas, J. Kuhlmann and H. Waldmann, unpublished results.
Footnote |
| † Electronic supplementary information (ESI) available: Cloning, expression and activation of H-Ras (1–180)-MESNA; ligation and purification of the chimeric Ras-proteins; preparation of lipid vesicles for SPR experiments. See DOI: 10.1039/b511736d |
| This journal is © The Royal Society of Chemistry 2006 |
