Separation of samarium and neodymium: a prerequisite for getting signals from nuclear synthesis
Samir
Maji
a,
Susanta
Lahiri
*b,
Birgit
Wierczinski
c and
Gunther
Korschinek
d
aDepartment of Chemistry, The University of Burdwan, Burdwan, 713104, India
bChemical Sciences Division, Saha Institute of Nuclear Physics, 1/AF Bidhannagar, Kolkata, 700064, India. E-mail: susanta.lahiri@saha.ac.in
cInstitut für Radiochemie, Technische Universität München, Walther-Meissner-Strasse 3 Garching, 85748, Germany
dTechnische Universität München, Fakultät für Physik, James-Franck-Straβe-1, Garching, 85747, Germany
First published on 28th September 2006
Abstract
146Sm (T1/2 = 108 y) is a long-lived radionuclide which has been produced in significant amounts during burning in a supernova (SN). Detection of this SN produced long-lived radionuclide on Earth may be helpful for getting information on nuclear synthesis at the time of our solar system’s formation. Only accelerator mass spectrometry (AMS) can determine such minute traces of 146Sm still expected in the Earth’s crust. However, the villain of 146Sm measurement through AMS is its naturally occurring stable isobar 146Nd which is a million times more abundant than the trace amount of 146Sm. Therefore an efficient method for the separation of samarium and neodymium is required to measure 146Sm through AMS. A simple liquid–liquid extraction (LLX) based method for separation of samarium and neodymium has been developed using radiometric simulation. Di-(2-ethylhexyl)phosphoric acid (HDEHP) has been used as the organic reagent. A very high separation factor (∼106) can be achieved when a solution containing samarium and neodymium is reduced by hydroxylamine hydrochloride followed by extraction with 0.1% HDEHP diluted in cyclohexane from 0.025 M HCl solution.
Introduction
The process that yields element formation during nuclear reactions in stars is called nuclear synthesis1 (detailed discussions can be found in textbooks such as ref. 1). A stellar process like a supernova (SN) is a source for nuclear synthesis that yields stable but also radioactive nuclides, which may end in new solar systems and planetary formation processes. Most of the unstable nuclides, present at the beginning in our solar system have faded away. But some of them with sufficiently long half-lives are still around, such as 238U. Measurement of specific radioactive nuclei on Earth synthesized before the formation of our solar system is a new approach to understanding these processes. 146Sm (T1/2 = 108 y), and 244Pu (T1/2 = 82.6 × 106 y)2 are such long-lived radionuclides, which have been produced in significant amounts and should still be around in measurable quantities because of their long half-lives. Also, short-lived nuclei can be found if formed during recent SNe close enough to our solar system to survive transportation to Earth. The recent finding of SN formed 60Fe (T1/2 = 1.5 × 106 y) in a deep sea ferromanganese crust3 supports this concept and showed the great potential of long lived radionuclides as a probe for searching astronomical events. Besides 60Fe, 182Hf (T1/2 = 9 × 106 y) is also of great interest,3 as it is a pure r-process nuclide. The r-process (r for rapid) occurs when elements (more massive than Fe) are under intense bombardment of neutrons, and the timescale for their decay by beta particles is long compared to the neutron capture. This enables reactions involving highly unstable intermediate nuclei. 182Hf is formed under such conditions in SNe.4Although accelerator mass spectrometry (AMS) is well suited for detecting minute traces of these radionuclides, sometimes the measurement itself is a big challenge because of the naturally occurring stable isobars. For example, any attempt to measure 182Hf by AMS must assure that the sample is free from its naturally occurring stable isobar 182W because the natural abundance of 182W is a million times more than the ultra-trace analyte 182Hf. Recently we have proposed a method to separate tungsten from hafnium as a prerequisite of 182Hf analysis by AMS.5 Similarly, up to now no measurement or attempted measurement has been performed to detect 146Sm in natural samples. The obstacle, until now, is the lack of a method to detect such low concentrations. Because of the long half-life, any attempt to count directly the decay of 146Sm is out of the scope of present detector technology. The measurement of 146Sm through AMS is also a very big challenge. Any attempt to measure 146Sm by AMS must ensure that the sample is free from its naturally occurring stable isobar 146Nd. Because of the natural abundance of 146Nd, its concentration might be a million times greater than the ultra-trace 146Sm. Again, elements samarium and neodymium, because of their similar chemical properties, always occur together in nature. The phenomenon of lanthanide contraction is mainly responsible for this similarity; introducing a colossal challenge for their mutual decontamination. Therefore as a first step of AMS detection of 146Sm, separation of samarium and neodymium is reported in this paper.
There are many reports on chromatographic techniques describing the separation of samarium and neodymium. Some of the recent results on samarium and neodymium separation by chromatographic techniques have been reported by Stray and Dahlgren,6 Arai et al.,7 Miranda Jr. et al.,8 Pin et al.,9 Schwantes et al.,10 Tsuyoshi and Akiba,11etc. However, the chromatographic methods require exhaustive instrumentation, chemical treatment and are time consuming. The sample size is less compared to liquid–liquid extraction (LLX) systems. The parameters like flow rate, temperature, gradient elution, etc., in chromatographic system are highly sensitive so that slight changes in the specified condition affect the separation heavily. Moreover, all chromatographic techniques reported so far offer only a narrow range of eluent volume where separation of Sm and Nd can be achieved. Compared to chromatographic techniques LLX is easy to handle, simple and much faster. A larger sample volume can be handled at a time. There are only a few reports on the separation of samarium and neodymium employing LLX. Rehkämper et al.12 attempted separation of Ce from other rare earth elements for application in Sm–Nd chronometry employing LLX using HDEHP as extractant. Their interest was on the primordial radionuclide 142Nd (an alpha decay product of 146Sm) for which 142Ce is the stable isobar. They were successful in separating Ce from the Sm–Nd pair but because of above mentioned interest they did not attempt mutual decontamination of Sm and Nd. Maharana and Nair13 reported separation of various rare earths by PC-88A (mono-2-ethylhexyl ester of 2-ethylhexyl phosphoric acid). Despite these reports, to the best of our knowledge, to date efficient separation of Sm and Nd with a very high separation factor through LLX has not been reported. The present paper reports the separation of Sm and Nd through LLX with the aim of achieving a high separation factor so that the result can be directly applied to decontaminate 146Sm from 146Nd for measurement of the former through AMS.
Experimental
To simulate the separation of Sm and Nd, we have irradiated Sm(NO3)3·5H2O (0.05 g) and Nd(NO3)3·6H2O (0.05 g) for 1 h and 1 day respectively by thermal neutrons with a flux of 1.3 × 1014 cm−2 s−1 in the FRM II reactor in Munich. Both salts were dissolved separately in 5 mL water and mixed together for separation studies. The amounts of samarium and neodymium at different stages of the experiment were monitored by the radioisotopes 153Sm (46.75 h) and 147Nd (10.98 d), respectively. All the chemical reagents used were of analytical grade. The reagents HDEHP, hydroxylamine hydrochloride, samarium nitrate and neodymium nitrate were procured from Merck, while cyclohexane was procured from Fluka.The separation studies were performed using liquid–liquid extraction (LLX). HDEHP was used as organic extractant and cyclohexane was used as diluent. Hydroxylamine hydrochloride was used to reduce Sm(III) to Sm(II). The extractant solutions of desired concentrations were prepared by adding calculated amounts of cyclohexane to HDEHP. In order to study the separation and extraction profile, 100 µL of mixed Sm and Nd solution was added to 10 mL of different concentration of HCl solutions and was shaken vigorously with an equal volume of HDEHP solution of the desired concentration for about 10 min. After disengagement of the liquid phases, 5 mL of aqueous as well as organic phase were taken separately in plastic vials and the counts were compared with a vertical HPGe detector (ORTEC) of 2.1 keV resolution at 1.33 MeV connected to a PC based multichannel analyzer. The activities present in both aqueous and organic phases were measured by the characteristic photo peaks of 153Sm (103.18 keV) and 147Nd (531.0 keV). The distribution coefficients as well as the percentage extraction of the elements were calculated. The extraction study was also carried out from HCl solution in presence of the reducing agent hydroxylamine. Back extraction was carried out with 0.1 M HCl. The stripped solution was diluted to 0.025 M HCl solution, and extracted again with 1% HDEHP in the presence of hydroxylamine.
Results and discussions
To separate Sm and Nd by liquid–liquid extractions, at first HDEHP concentrations were varied keeping the HCl concentration fixed at 0.01 M (Table 1). It was observed that extractions of both Sm and Nd increased with increasing HDEHP concentration and always with the same quantum. Therefore, no separation was found at higher HDEHP concentration. However, at lower concentration, i.e. at 0.1% HDEHP concentration, some difference in the extraction behavior of Sm and Nd was observed. Therefore all the subsequent experiments were carried out with HDEHP concentration fixed at 0.1%.| HCl | [HDEHP] (%) | K D(Sm) | K D(Nd) | SF |
|---|---|---|---|---|
| 0.01 M | 0.10% | 1.651 ± 0.0161 | 0.1372 ± 0.0084 | 12.03 |
| 1% | 330.8 ± 22.89 | 284.5 ± 104.8 | 1.163 | |
| 2% | 382.8 ± 29.16 | 348.7 ± 149.1 | 1.098 | |
| 4% | 440.1 ± 35.66 | 405.3 ± 196.8 | 1.086 |
Fig. 1 shows the extraction profile of Sm and Nd against varying HCl concentration. It has been found that distribution coefficients of both samarium and neodymium are in general low and decrease with increasing acid strength. At lower acidity the slightly higher extraction of samarium and neodymium might be attributed to the formation of cationic aqua-complexes like [Ln(H2O)x]3+ or [Ln(OH)]2+, which in turn form complexes with the cationic extractant HDEHP14. Fig. 1 also shows that there is no encouraging variation in the extraction behavior of samarium and neodymium so that they can be separated from each other for AMS measurement.
 | ||
| Fig. 1 Distribution coefficients (KD) of Sm and Nd in varying HCl concentrations at fixed 0.1% HDEHP concentration | ||
As samarium also exhibits a +2 oxidation state, hydroxylamine hydrochloride was added to the mixture of radioactive solution of samarium and neodymium and the same experiment was repeated again. Fig. 2 shows the extraction profile of 153Sm and 147Nd in the presence of hydroxylamine hydrochloride against varying HCl concentration keeping HDEHP concentration fixed at 0.1%. After addition of hydroxylamine hydrochloride the KD values of both Sm and Nd increased many times, though increase of KD values of samarium is much higher than Nd. The increase in extraction may be attributed to the fact that both Sm and Nd are reduced to the +2 state, resulting in an increase in ionic radii. Therefore, extraction into the organic phase can occur not only by an ion exchange mechanism but also through Ln(DEHP)2 complexes. The +2 state of Nd is not stable and therefore KD values of Nd are smaller than those of Sm. At optimal conditions, i.e. at 0.025 M HCl and 0.1% HDEHP in the presence of hydroxylamine hydrochloride, a separation factor as high as ∼106 has been achieved (Table 2). However, at this condition ∼4.65% Nd is extracted along with 100% Sm into the organic phase. To get rid of this neodymium contamination, the metals extracted in the organic phase are stripped back completely into the aqueous phase with the help of 0.1 M HCl. The aqueous phase was diluted to 0.025 M HCl and the process of extraction was repeated again. Table 2 shows the percentage extraction of Sm and Nd as well as KD values of Sm and Nd after repeating the extraction–back extraction cycle twice. Fig. 3 schematically presents the developed method for the separation of Sm and Nd.
 | ||
| Fig. 2 Distribution coefficients (KD) of Sm and Nd in varying HCl concentrations at fixed 0.1% HDEHP concentration in presence of hydroxylamine. | ||
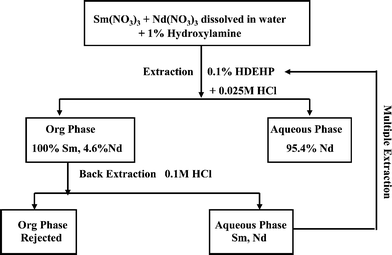 | ||
| Fig. 3 Schematic diagram for separation of Sm and Nd. | ||
| Conditions | K D(Sm) | K D(Nd) | SF | Sm (%) | Nd (%) | |
|---|---|---|---|---|---|---|
| 1st extraction | 0.025M HCl + 0.1% HDEHP + 1% NH2OH,HCl | 4.641 × 104 ± 215.4 | 0.0489 ± 0.0011 | 9.498 × 105 | 100.0 ± 0.9240 | 4.659 ± 0.1060 |
| 2nd extraction | 4.620 × 104 ± 214.9 | 0.0467 ± 0.0051 | 9.902 × 105 | 99.54 ± 0.9208 | 0.209 ± 0.0193 | |
| 3rd extraction | 4.599 × 104 ± 214.5 | 0.0397 ± 0.0234 | 1.159 × 106 | 99.09 ± 0.9177 | 0.008 ± 0.0037 |
Conclusion
The developed process for the separation of samarium and neodymium not only offers a very high separation factor but also ensures suppression of neodymium contamination from samarium greater than 106 times after multiple extraction and back extraction processes. The rest of the separation of 146Nd versus146Sm can be done by AMS. Therefore, the developed method will be highly helpful for pretreatment of samarium samples for AMS measurement of ultra-trace amounts of 146Sm in nature to study nuclear synthesis.Acknowledgements
The authors gratefully acknowledge the Department of Science and Technology (DST), India and DAAD, Germany.References
- J. M. Pasachoff, Astronomy: From the Earth to the Universe, Sounders College Publishing, New York, 5th edn, 1998, ISBN 0-03-024347-5 Search PubMed.
- C. Wallner, T. Fastermann, U. Gersmann, K. Knie, G. Korschinek, C. Lierse and G. Rugel, New Astron. Rev., 2004, 48, 145–150 CrossRef CAS.
- K. Knie, G. Korschinek, T. Faestermann, E. A. Dorfi, G. Rugel and A. Wallner, Phys. Rev. Lett., 2004, 93.
- C. Vockenhuber, C. Feldstein, M. Paul, N. Trubnikov, M. Bichler, R. Golser, W. Kutschera, A. Priller, P. Steier and S. Winkler, New Astron. Rev., 2004, 48, 161 CrossRef CAS.
- S. Maji, S. Lahiri, B. Wierczinski and G. Korschinek, Anal. Chem., 2006, 78, 2302 CrossRef CAS.
- H. Stray and S. Dahlgren, Chem. Geol., 1995, 125, 233–238 CrossRef CAS.
- T. Arai, Y. Wei, M. Kumagai and K. Horiguchi, J. Alloys Compd., 2006, 408–412, 1008 CrossRef CAS.
- P. Miranda, Jr., M. F. Máduar, G. Vicentini, L. B. Zinner, N. M. P. Moraes and H. M. Shihomatsu, J. Alloys Compd., 2002, 344, 46–50 CrossRef.
- C. Pin and J. F. S. Zalduegui, Anal. Chim. Acta, 1997, 339, 79–89 CrossRef CAS.
- J. M. Schwantes, R. S. Rundberg, W. A. Taylor and D. J. Vieira, J. Alloys Compd., 2006, 418, 189–194 CrossRef CAS.
- A. Tsuyoshi and K. Akiba, Anal. Sci., 2000, 16, 843 CAS.
- M. Rehkämper, M. Gärtner, S. J. G. Galer and S. L. Goldstein, Chem. Geol., 1996, 129, 201 CrossRef.
- L. M. Maharana and V. R. Nair, Light Met., 2005, 1163 Search PubMed.
- D. Nayak, S. Lahiri and N. R. Das, J. Radioanal. Nucl. Chem., 1999, 240, 555 CAS.
| This journal is © The Royal Society of Chemistry 2006 |
