Improving the biocompatibility of in vivo sensors via nitric oxide release
Jae Ho
Shin
and
Mark H.
Schoenfisch
*
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-3290, USA. E-mail: schoenfi@email.unc.edu; Fax: +1 919 962 2388; Tel: +1 919 843 8714
First published on 27th March 2006
Abstract
The continuous, real-time monitoring of clinically important analytes (e.g., PO2, PCO2, pH, K+, Na+, glucose, and lactate) is of great importance to human health care. Despite considerable efforts spanning several decades, the use of in vivo sensors clinically remains limited due to inadequate biocompatibility. The discovery of nitric oxide (NO) as an effective inhibitor of platelet and bacterial adhesion has opened a new direction of research related to designing the next generation of in vivo sensors. In this Highlight article, recent progress in designing more biocompatible in vivo sensors is described, with a particular focus on preparing interfaces that resist biofouling via controlled NO release.
1. In vivo sensor biocompatibility
The demand for implantable chemical sensors capable of monitoring the physiological status of patients on a continuous, real-time basis (i.e., in the presence of biological milieu) continues to be of great importance in human health care.1–3In vivo sensors must operate reliably, rapidly, and selectively over extended periods (hours to months) under harsh conditions. Despite considerable efforts, designing in vivo sensors for clinical use remains a major challenge due to compromised sensor performance upon implantation and severe health risks to the implant recipient. These serious obstacles result primarily from undesirable interactions between the sensor surface and biological medium.2,3 Indeed, the insertion of a foreign material into the body brings about immediate physiological responses that are dependent upon the location of indwelling probes (i.e., blood, tissue, etc.).Examples of blood-mediated biofouling include protein deposition, platelet adhesion/activation, and surface-induced thrombosis. Such biofouling often plagues intravascular sensors.2,4 The adsorption of plasma proteins (e.g., fibrinogen and von Willebrand factor) on the implant surface is the first stage of the surface fouling process. Platelets adhere to surface-bound proteins via the expression of platelet receptors (e.g., glycoprotein IIb/IIIa), and then spread on the surface to form aggregates with adjacent platelets via interactions with fibrinogen and other plasma ligands. Platelet aggregation triggers structural changes in the organization of the surface membrane, leading to exposure of a highly procoagulant lipid surface to blood, which facilitates thrombin activation. Thrombin then serves to convert fibrinogen to a polymeric fibrin mesh, resulting in the accumulation of a dense network of fibrin, platelets, and entrapped blood cells, known as a thrombus (blood clot). Such surface fouling leads to significant deterioration in sensor reliability and/or longevity. The adsorption of metabolically active cells (e.g., platelets) alters the chemical environment adjacent to the implant via consumption of O2 and production of CO2, subsequently lowering the local or measured pH. Furthermore, normal blood flow may be inhibited by the presence of the thrombus and/or constriction of the vascular walls around the implant, ultimately increasing the risk of thromboembolism.
Sensors implanted in the subcutaneous tissues are more susceptible to cell adhesion and fibrous tissue encapsulation in response to the body's self-defense mechanism.3,5 Upon implantation, tissue is inherently disrupted and capillaries damaged, concomitantly initiating a wound healing cascade (i.e., immune response). The wound healing cascade consists of four distinct stages that are expressed at different times (ranging from seconds to weeks): hemostasis, inflammation, repair, and encapsulation. The acute inflammatory response takes place within seconds after the sensor is implanted. During this stage, proteins and inflammatory cells adsorb to the sensor surface. Phagocytic cells (e.g., neutrophils, monocytes, and macrophages) then surround the device (minutes to hours later) and attempt to destroy it. Such membrane biofouling is detrimental to sensor function resulting in restriction of analyte diffusion to the sensor and/or degradation of the sensor membrane. Macrophages adsorbed closely to the sensor surface also perturb local concentrations of analytes due to the consumption of glucose and O2, and the release of reactive oxygen species (e.g., H2O2, O2−, NO, and OH−). During the repair or wound healing stage (occurring days later), the tissue surrounding the implant becomes increasingly avascular as the body attempts to encapsulate and isolate the foreign material. Encapsulation further aggravates the flux of analyte to the sensor surface.
An equally challenging problem affecting in vivo sensors to be implanted for extended periods is bacterial adhesion which often leads to infection with serious risks to implant recipients.6,7 Despite sterilization and aseptic procedures, bacterial infection remains a great impediment to the utility of all indwelling devices. Although helpful in minimizing bacteria, sterilization is often destructive (e.g., polymer degradation) and does not eliminate the risk of bacterial infection. Implant-associated infections are commonly caused by microbial biofilms that form around the implant. The initial adhesion of individual bacterial cells is followed by cell association and proliferation to form aggregates or microcolonies that secrete a polymeric shield consisting of exopolysaccharides. Once formed, biofilms are formidable physiological and chemical barriers, impeding both phagocytosis by the body's immune system and the action of conventional antibiotic therapies. As a result, implant recipients often suffer from serious and persistent infections that necessitate removal of the implanted device.
Strategies to address surface biofouling have focused on developing more biocompatible polymer coatings. Indeed, several polymeric materials including hydrogels, phospholipid-based biomimicry, Nafion, surfactant-derived membranes, diamond-like carbons, polyurethane, and silicone elastomers, have been used to modify the sensor surface to passively suppress protein and cell adhesion.8 To date, these approaches have not proven effective clinically (i.e., in vivo). Strategies based on the use of more “active” biomaterials designed to controllably release an anticoagulant (e.g., heparin)9 or antimicrobial agent (e.g., antibiotics and polyclonal antibodies)10 at the implant site have thus been pursued. While anticoagulant treatment may be useful for preventing thrombus formation at the blood-material interface, it may also lead to undesirable systemic effects (e.g., uncontrolled bleeding), a serious problem for patients who suffer from medical conditions such as hemophelia, renal failure, or thrombocytopenia that affect blood clotting. Furthermore, antibiotic treatments for reducing bacteria-related infections are becoming less effective due to antibiotic resistance among pathogens and the related side effects often associated with such drugs. Consequently, the achievement of a truly biocompatible sensor has not yet been realized, despite significant advances in designing synthetic polymers for in vivo sensor applications.
2. Nitric oxide and prevention of biofouling
2.1. Biological roles of nitric oxide and potential implications
Nitric oxide (NO) is a diatomic free radical endogenously synthesized in the human body when L-arginine is converted to L-citrulline by a class of enzymes known as nitric oxide synthases (NOS's).11,12 Since the first reports describing NO's action as an endothelium-derived relaxation factor (EDRF) in the middle of 1980s, research efforts have been devoted to elucidating the pathways of NO generation and action in vivo. To date, researchers have discovered that NO regulates a range of crucial biological processes in the cardiovascular, gastrointestinal, genitourinary, respiratory, and central and peripheral nervous systems. The relatively recent discovery of NO as a potent inhibitor of platelet adhesion and activation,13 and its identification as both an antimicrobial agent14 and angiogenic factor15 have extended NO research to the field of biomaterials.The non-thrombogenic properties of the vascular endothelium are primarily attributed to NO.2,11,16 Indeed, both basal and stimulated endothelial cells continuously release NO into the lumen of blood vessels at an estimated flux of 0.5–1.0 × 10−10 mol cm−2 min−1.11 The NO released regulates blood flow and pressure, and prevents platelet aggregation under normal conditions. The generation of NO by macrophages has also been implicated in fighting invading microorganisms.11 Although NO's action in wound healing is not fully understood, NO may also mediate immune and inflammatory responses. Monocytes and macrophages stimulated by foreign cells (e.g., bacteria) produce NO to destroy such cells via pathways mediated by NO and its reactive intermediates (e.g., peroxynitrite and dinitrogen trioxide). Finally, NO plays a role in angiogenesis or the formation and growth of new blood vessels.11 With respect to subcutaneous implant applications, NO may allow for increased blood flow to the sensor surface, thus enhancing mass transfer of analyte, while reducing bacterial adhesion and associated infection risks.
In addition to NO's numerous physiological functions, the effects of NO are often localized due to its short half-life (ranging from 1 s to a few minutes depending on the concentration of oxygen and the presence of NO scavengers such as oxyhemoglobin) in biological milieu.11 For example, NO released from the surface of a sensor would be rapidly consumed via reactions with oxygen and other scavengers (e.g., hemoglobin and thiols). Thus, certain health risks associated with systemic administration of anticoagulants and antibiotics may be avoided by employing NO-release coatings.
2.2. Synthetic nitric oxide donors and polymers
A number of synthetic NO donors including nitrosothiols, nitrosamines, diazeniumdiolates, metal complexes, and organic nitrates/nitrites have been used to design polymer coatings capable of slowly releasing therapeutic levels of NO that are effective in reducing biofouling.17 Of these NO donor species, 1-amino-substituted diazen-1-ium-1,2-diolates or more simply “N-diazeniumdiolates” have emerged as attractive candidates for designing more biocompatible coatings due to their ability to generate NO spontaneously under physiological conditions.18,19 Since the first report on the synthesis of N-diazeniumdiolates by Drago and Paulik in 1960,20 several diazeniumdiolate species have been synthesized using a range of nucleophile residues that encompass simple primary/secondary amines, polyamines, and secondary amino acids.18 Keefer and coworkers have reviewed the synthesis of these nucleophilic NO adducts via the reaction of polyamines with NO at elevated pressure.18 Briefly, one equivalent of amine (N) reacts with two equivalents of NO (Scheme 1). A second equivalent of base (e.g., another amine) is then protonated to sustain the newly formed [N(O)NO]− group, yielding zwitterionic stabilized structures. While stable under ambient conditions, N-diazeniumdiolates decompose spontaneously in aqueous media to generate NO at rates dependent upon pH, temperature, and/or the structure of the amine moiety. | ||
| Scheme 1 Reaction of NO with amines to produce N-diazeniumdiolate NO donors followed by the subsequent generation of NO in the presence of water. | ||
The original approach for preparing NO-releasing polymers was to incorporate or disperse small molecule N-diazeniumdiolate NO donors into a polymer during synthesis of the polymer. Espadas-Torre et al. demonstrated the utility of such NO-releasing polymers to prepare potentiometric ion sensors for the measurements of potassium (K+) and hydrogen (H+).21 Plasticized poly(vinyl chloride) (PVC) and polyurethane membranes were doped with an appropriate ionophore (i.e., valinomycin for K+ and tridodecylamine for H+, respectively) and (Z)-1-(N-methyl-N-[6-(N-methylammoniohexyl)amino]diazen)-1-ium-1,2-diolate or diazeniumdiolated N,N′-dimethyl-1,6-hexanediamine (MAHMA/N2O2). Schoenfisch et al. subsequently reported on a Clark-style amperometric oxygen-sensing catheter using a MAHMA/N2O2-doped silicone rubber (SR) polymer film as a gas-permeable membrane.22 These intravascular sensors generated NO upon exposure to aqueous solutions (e.g., blood) and exhibited significantly improved in vitro and in vivo hemocompatibility without impaired analytical performance. Unfortunately, the non-covalently entrapped NO donors (e.g., MAHMA/N2O2) and their carcinogenic decomposition byproducts (e.g., diamines and corresponding nitrosamines) were found to leach from the polymer matrix into the surrounding media.23
To address leaching concerns, efforts were devoted to (1) utilize more lipophilic NO donors that would preferentially remain in the organic polymer phase; and, (2) covalently tether the NO donor agents to the polymer backbone. Batchelor et al. increased the lipophilicity of the original NO donor MAHMA/N2O2 by using longer alkyl chains (i.e., diazeniumdiolated N,N′-dibutylhexanediamine; DBHD/N2O2).24 A catheter-type amperometric oxygen sensor coated with a hydrophobic SR membrane containing such lipophilic NO donors (i.e., DBHD/N2O2) was then fabricated and evaluated in vivo.16 The NO-release properties of the sensor were controllable by varying the amount of NO donors blended into the polymer casting solution. Both leaching of the NO donor and platelet aggregation were significantly reduced. In an alternative approach, the N-diazeniumdiolate moiety has been covalently attached to the backbone of the polymer. For example, the synthesis of diazeniumdiolated polymethacrylate, piperazine-modified PVC, poly(ethyleneimine), diamine-modified SR, and polyurethane has been reported.23,25,26 Mowery et al. demonstrated the fabrication of K+- and H+-selective membrane electrodes using diazeniumdiolated poly(ethyleneimine) (PEI/N2O2) and methoxymethyl-protected diazeniumdiolated piperazine-PVC (mompipPVC/N2O2).27 Hydrophobic silicone materials were similarly synthesized by cross-linking N-(6-aminohexyl)-3-aminopropyltrimethoxysilane (DACA-6) with poly(dimethylsiloxane) (PDMS), followed by forming N-diazeniumdiolates in situ (DACA-6/N2O2-SR).28 DACA-6/N2O2-SR films also have been successfully employed as NO-releasing outer coatings for the fabrication of amperometric28 and fluorescence-based oxygen sensors,29 and a potentiometric carbon dioxide-sensing catheter.16
2.3. Sol–gel chemistry for nitric oxide-releasing coatings
Our laboratory has focused on the synthesis and characterization of sol–gel derived materials (i.e., xerogels) whereby the N-diazeniumdiolate NO donors are covalently bound to the xerogel backbone.15,30–33 These materials represent attractive sensor coatings because they combine the utility of tunable NO release with the versatility of sol–gel chemistry. In particular, xerogels have emerged as a class of materials suitable for a wide range of sensing applications since they are: (1) synthesized under mild conditions; (2) chemically flexible; and, (3) generally porous, thus facilitating the diffusion of analyte to the transducer (usually an electrode). Sol–gel derived films also exhibit strong adhesion to a variety of sensor substrates (e.g., metal/metal oxides and silica substances).A range of inorganic–organic hybrid xerogels functionalized to release NO have been synthesized using sol–gel chemistry.34 In a typical procedure, alkyl- and aminosilane precursors are mixed with appropriate amounts of water, methanol or ethanol, and a catalyst (e.g., acid or base). The silane precursors are hydrolyzed, resulting in the formation of silanol groups (Si–OH) (Scheme 2a). The reactive silanols then cross-react (i.e., condense) with either alkoxy (Si–OR) or other silanol groups to yield a siloxane bridge (Si–O–Si) where R is typically a methyl or ethyl group, and R′ is an amine moiety (Scheme 2b). Eventually, polycondensation reactions lead to the formation of a polymeric gel network. Subsequently, the amine functional groups in the cured xerogel structure are converted to N-diazeniumdiolate NO donors via exposure to high pressures of NO. Analogues to the polymers described above, the generation of NO from diazeniumdiolated xerogels is triggered by the presence of a proton donor such as water (Fig. 1). A range of diazeniumdiolate-modified xerogels have been synthesized and their properties tailored by varying the type and amount of alkyl- and aminosilane precursors and specific reaction/processing conditions (e.g., pH, catalyst, water content, and drying time and temperature). Several alkyl- and aminosilane precursors were evaluated for this purpose, including methyl-, ethyl-, and butyltrimethoxysilanes (MTMOS, ETMOS, and BTMOS, respectively), (aminoethylaminomethyl)phenethyltrimethoxysilane (AEMP3), N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (AEAP3), N-(6-aminohexyl)aminopropyltrimethoxysilane (AHAP3), and N-[3-(trimethoxysilyl)propyl]diethylenetriamine (DET3). The chemical flexibility allowed via sol–gel chemistry can be further manipulated by doping other molecules (e.g., biomolecules, polymers, and electrochemical or optical sensing elements) within the xerogel network.
 | ||
| Fig. 1 Schematic of NO generation from N-diazeniumdiolate-modified xerogel network occurring upon exposure to aqueous conditions. | ||
 | ||
| Scheme 2 Schematic of sol–gel process illustrating (a) hydrolysis of silane precursors and (b) subsequent condensation reactions where R is typically a methyl or ethyl group and R′ is an amine moiety for preparing aminosilane-based xerogels. | ||
3. Recent progress in nitric oxide-releasing sensors
Monitoring of blood glucose levels is of great importance for effectively managing diabetes.3 Continuous, real-time monitoring of glucose would be particularly beneficial in alerting someone with diabetes to hypoglycemia (i.e., low glucose levels) that often lead to unconsciousness and other undesirable complications. Since such glucose sensors must function in vivo for extended periods, they are more likely to be placed under the skin (subcutaneously), rather than into the blood vessel (intravascularly). While advances in the synthesis of NO-releasing polymers have led to significant progress in the development of intravascular sensors for blood gas and electrolyte measurements,2,16 the application of such coatings to subcutaneous glucose sensors may also prove beneficial.Shin et al. reported on the feasibility of combining NO release with enzymatic glucose sensing.35 Notably, the analytical response of aminosilane-based xerogels doped with glucose oxidase (GOx) was significantly lower than controls (reduced by 99.8%) upon exposure to NO gas (for diazeniumdiolate synthesis). Such behavior was attributed primarily to hindered glucose diffusion through the xerogel film as a result of enhanced polycondensation catalyzed by NO.35,36 To overcome this serious limitation, N-diazeniumdiolate-modified xerogel particles were physically entrapped within hydrophobic polyurethane membranes. In this manner, the GOx-doped xerogel layer was not exposed to the reactive, harsh conditions necessary to impart NO release. The resulting sensor consisted of a platinum (Pt) electrode modified with four polymeric membranes: (1) a xerogel (i.e., MTMOS) layer with immobilized GOx; (2) a polyurethane coating designed to protect the enzyme from undesirable diffusion of NO; (3) a polyurethane layer containing NO-releasing xerogel particles; and, (4) a polyurethane outer film (Fig. 2). The outermost polyurethane barrier served to prevent leaching of xerogel particles and control glucose diffusion relative to oxygen, maximizing the sensor's dynamic response range. Of the xerogel combinations studied, 40% AHAP3 (v/v, balance MTMOS) xerogel particles exhibited the greatest levels of NO release for the longest periods. Unfortunately, particles prepared with larger aminosilane concentrations (>40%, v/v in total silane) were unstable upon immersion in aqueous solution. The NO-releasing xerogel particle/polyurethane hybrid sensor was characterized by ample sensitivity, a wide linear range (1 to 31 mM of glucose), and rapid response times (ca. 20 s). In vitro bacterial adhesion assays indicated that the NO flux generated from the sensor surface was sufficient to reduce Pseudomonas aeruginosa adhesion.
 | ||
| Fig. 2 Schematic of the hybrid xerogel/polyurethane glucose biosensor employing N-diazeniumdiolate-modified xerogel particles supported in a polyurethane matrix. Reproduced from ref. 35 with permission. | ||
Alternatively, Oh et al. demonstrated the fabrication of a miniaturized needle-type glucose biosensor patterned with an N-diazeniumdiolate-modified xerogel microarray for overcoming the reduced analyte permeability observed with xerogel films.37 The biosensor design consisted of: (1) GOx immobilized within a MTMOS xerogel; (2) a blended polyurethane/hydrophilic polyurethane membrane that served to prevent enzyme leaching and impart selectivity for glucose; and, (3) micropatterned xerogel lines (5 µm wide separated by 20 µm), allowing for NO release (Fig. 3). This configuration allowed for enhanced glucose sensitivity relative to sensors modified with xerogel films since significant portions of the underlying electrode surface remain unmodified. The diffusion of glucose to the GOx layer was thus less inhibited. Although the amperometric response of the sensor was reduced by 38% relative to the response before NO exposure, a rapid (ca. 30 s) and wide linear response (1 to 20 mM of glucose) was observed, indicating that the enzymatic activity of GOx was not significantly compromised by NO release. Xerogel micropatterning was also successfully employed to prepare an intravascular amperometric oxygen sensor.38 Comparisons between electrodes modified with xerogel films and micropatterns indicated that the accessibility of oxygen to the surface was increased by a factor of 20 (when microstructures were separated by 20 µm). The micropatterned NO-releasing glucose and oxygen sensors generated sufficient levels of NO to reduce both bacterial and platelet adhesion: 70–80% and ca. 90% decreases in P. aeruginosa and platelet surface coverage, respectively, were observed (Fig. 4).37,38 However, the NO release longevity from such patterns was limited (<48 h), ultimately leading to increased platelet and bacterial adhesion at extended periods. More recently, in vitro cell adhesion experiments were expanded to include other clinically relevant bacterial species including Proteus mirabilis, Staphylococcus aureus, and Staphylococcus epidermidis.39,40 Similar to P. aeruginosa, NO-releasing xerogel coatings inhibited cell adhesion to these pathogens (50–90% reduction) as well.
 | ||
| Fig. 3 Schematic of (a) planar sensing tip prior to surface modification and (b) the enzyme-based amperometric glucose biosensor micropatterned with an N-diazeniumdiolate-modified xerogel microarray. Reproduced from ref. 37 with permission. | ||
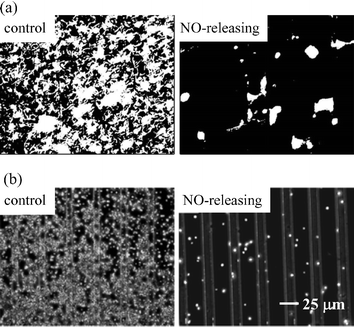 | ||
| Fig. 4 (a) Fluorescent optical images (200 × 165 µm) of P. aeruginosa adhesion and (b) phase contrast images of platelet adhesion to control and NO-releasing xerogel microarrays (5 or 10 µm wide separated by 20 or 25 µm). Bacteria and platelets are shown in white. Reproduced from ref. 37 and 38 with permission. | ||
An amperometric sol–gel derived sensor that releases NO and proved useful for measuring physiologically relevant concentrations of oxygen was reported by Marxer et al.41 The sensor consisted of a platinum electrode coated with 20% AHAP3 (balance ETMOS) hybrid xerogel film. Hydrophilic polyurethane (HPU) was doped into the xerogel membrane to increase oxygen permeability and reduce the time required to hydrate the membrane. N-Diazeniumdiolate NO donors were formed within the polymer matrix by exposing the cured film to high pressures of NO. The design of this sensor was straightforward and did not require multilayered configurations or micropatterned arrays. The coating released up to 4.3 × 10−10 mol cm−2 min−1 of NO over the first 12 h and maintained measurable levels of NO release through 48 h. Oxygen sensors modified with HPU-doped, NO-releasing xerogels effectively inhibited platelet adhesion without serious deterioration in sensor performance. In addition, the HPU-doped xerogel coating was stable in buffer solution for over 48 h.
Wilson and coworkers recently demonstrated the fabrication of a NO-releasing subcutaneous glucose biosensor.42 Both in vitro and in vivo testing including an evaluation of the inflammatory response at the implant/tissue (of normal non-diabetic Sprague-Dawley rats) interface for 3 d following implantation was conducted to assess the biocompatibility of such sensors. The sensor was prepared by coating a needle-type amperometric electrode with an outer polyurethane/PDMS membrane containing a lipophilic N-diazeniumdiolate NO donor species (i.e., DBHD/N2O2). A Clarke-error grid correlation3 between sensor glycemia estimates and blood glucose levels measured in Sprague–Dawley rats yielded 99.7 and 96.3% of points for the NO-releasing sensors controls, respectively, within clinically acceptable zones (A and B) on Day 1. In addition, a similar correlation was obtained on Day 3. Histological examination of the implant site at Day 1 demonstrated that the inflammatory response to the NO-releasing sensors was decreased by almost 100%. The suppressed inflammatory response was attributed to an increase of protein nitration in tissue surrounding the sensor.3 Notably, the sensor also responded to NO at a potential of +0.6 V (vs. Ag/AgCl), thus resulting in positive errors. (Nitric oxide is an electroactive species which is oxidized at +0.7 to +0.9 V vs. Ag/AgCl.)36 Although this did not appear to affect the accuracy of the NO-releasing glucose sensors because the concentration of NO released was small, the authors implied that NO released from inflammatory cells may impact in vivo sensor performance.
4. Conclusions and future directions
Understanding and minimizing surface-induced platelet aggregation and bacterial adhesion are critical for improving the biocompatibility of blood and subcutaneous sensors. A unique strategy for preparing interfaces that resist biofouling and maintain more optimal functionality has been introduced via NO-releasing membranes. The flux of NO released from N-diazeniumdiolate-modified polymers is sufficient to reduce surface fouling. Indeed, numerous in vitro and in vivo studies have revealed that employing such NO-releasing coatings may represent a powerful strategy for combating implant-related biofouling.S-Nitrosothiols represent alternative NO donors for preparing NO-releasing polymers. For example, Bohl and West reported the synthesis and characterization of S-nitrosocysteine-linked polyethylene glycol hydrogels.43 Such coatings were shown to reduce platelet adhesion and smooth muscle cell proliferation. In separate studies, Frost and Meyerhoff reported on the photolytic release of NO from S-nitroso-N-acetyl-DL-penicillamine derivatized fumed silica particles.44,45 Fumed silica is easily blended into polymers and thus could be used to fabricate NO-releasing sensors.
Despite the benefits of NO release, some limitations remain. Specifically, the NO-release longevity of such coatings is limited by the finite reservoir of NO donors that may be loaded into a thin film. Employing polymeric coatings that utilize endogenous NO donor species to continuously generate therapeutic levels of NO at the sensor interface may represent a better approach for enhancing NO-release capability long term. Oh et al. recently reported on the utility of doping polymers with biomimetic lipophilic copper catalysts for achieving continuous conversion of nitrite to NO in the presence of physiological levels of endogenous reducing agents (e.g., ascorbic acid, cystein, or glutathione).46,47 Efforts for combining such biomimic strategies with chemical sensing techniques are currently underway.
Acknowledgements
The authors gratefully acknowledge support from the National Institutes of Health (NIH Grant EB000708).References
- G. S. Wilson and Y. Hu, Chem. Rev., 2000, 100, 2693 CrossRef CAS.
- M. C. Frost and M. E. Meyerhoff, Curr. Opin. Chem. Biol., 2002, 6, 633 CrossRef CAS.
- G. S. Wilson and R. Gifford, Biosens. Bioelectron., 2005, 20, 2388 CrossRef CAS.
- J.-P. Cazenave and J. Mulvihill, The Role of Platelets in Blood-Biomaterial Interactions, ed. Y. F. Missirlis and J.-L. Wautier, Kluwer, Dordrecht, 1993 Search PubMed.
- Handbook of Biomaterials Evaluation: Scientific, Technical, and Clinical Testing of Implant Materials, ed. A. F. von Recum, Taylor & Francis, Philadelphia, 1998 Search PubMed.
- J. Dankert, A. H. Hogt and J. Feijen, CRC Crit. Rev. Biocompat., 1986, 2, 219 Search PubMed.
- Antimicrobial/Anti-Infective Materials: Principles, Applications and Device, ed. S. P. Sawan and G. Manivannan, Technomic Publishing Co., Lancaster, 2000 Search PubMed.
- N. Wisniewski and M. Reichert, Colloids Surf., B, 2000, 18, 197 CrossRef CAS.
- J.-C. Huang and E. M. Jennings, Int. J. Polym. Mater., 2004, 53, 69 Search PubMed.
- S. K. Hendricks, C. Kwok, M. Shen, T. A. Horbett, B. D. Ratner and J. D. Bryers, J. Biomed. Mater. Res., 2000, 50, 160 CrossRef CAS.
- L. J. Ignarro, Nitric Oxide: Biology and Pathobiology, Academic Press, San Diego, 2000 Search PubMed.
- A. R. Butler and R. Nicholson, Life, Death and Nitric Oxide, Royal Society of Chemistry, Cambridge, 2003 Search PubMed.
- M. W. Radomski, R. M. J. Palmer and S. Moncada, Br. J. Pharmacol., 1987, 92, 639.
- M. Ziche, E. Morbidelli, E. Masini, S. Amerini, H. J. Granger, C. Maggi, P. Geppetti and F. Ledda, J. Clin. Invest., 1994, 94, 2036 CrossRef CAS.
- B. J. Nablo, T.-Y. Chen and M. H. Schoenfisch, J. Am. Chem. Soc., 2001, 123, 9712 CrossRef CAS.
- M. C. Frost, M. M. Batchelor, Y. Lee, H. Zhang, Y. Kang, B. K. Oh, G. S. Wilson, R. Gifford, S. M. Rudich and M. E. Meyerhoff, Microchem. J., 2003, 74, 277 CAS.
- P. G. Wang, M. Xian, X. Tang, X. Wu, Z. Wen, T. Cai and A. J. Janczuk, Chem. Rev., 2002, 102, 1091 CrossRef CAS.
- J. A. Hrabie and L. K. Keefer, Chem. Rev., 2002, 102, 1135 CrossRef CAS.
- M. C. Frost, M. M. Reynolds and M. E. Meyerhoff, Biomaterials, 2005, 26, 1685 CrossRef CAS.
- R. S. Drago and F. E. Paulik, J. Am. Chem. Soc., 1960, 82, 96 CrossRef CAS.
- C. Espadas-Torre, V. Oklejas, K. Mowery and M. E. Meyerhoff, J. Am. Chem. Soc., 1997, 119, 2321 CrossRef CAS.
- M. H. Schoenfisch, K. Mowery, M. V. Rader, N. Baliga, J. A. Wahr and M. E. Meyerhoff, Anal. Chem., 2000, 72, 1119 CrossRef CAS.
- K. Mowery, M. H. Schoenfisch, J. E. Saavedra, L. K. Keefer and M. E. Meyerhoff, Biomaterials, 2000, 21, 9 CrossRef CAS.
- M. M. Batchelor, S. L. Reoma, P. S. Fleser, V. K. Nuthakki, R. E. Callahan, C. J. Shanley, J. K. Politis, J. Elmore, S. I. Merz and M. E. Meyerhoff, J. Med. Chem., 2003, 46, 5153 CrossRef CAS.
- P. G. Parzuchowski, M. C. Frost and M. E. Meyerhoff, J. Am. Chem. Soc., 2002, 124, 12182 CrossRef CAS.
- H. Zhang, G. M. Annich, J. Miskulin, K. Osterholzer, S. I. Merz, R. H. Bartlett and M. E. Meyerhoff, Biomaterials, 2002, 23, 1485 CrossRef CAS.
- K. Mowery, M. H. Schoenfisch, N. Baliga, J. A. Wahr and M. E. Meyerhoff, Electroanalysis, 1999, 11, 681 CrossRef CAS.
- M. C. Frost, S. M. Rudich, H. Zhang, M. A. Maraschio and M. E. Meyerhoff, Anal. Chem., 2002, 74, 5942 CrossRef CAS.
- M. H. Schoenfisch, H. Zhang, M. C. Frost and M. E. Meyerhoff, Anal. Chem., 2002, 74, 5937 CrossRef CAS.
- S. M. Marxer, A. R. Rothrock, B. J. Nablo, M. E. Robbins and M. H. Schoenfisch, Chem. Mater., 2003, 15, 4193 CrossRef CAS.
- B. J. Nablo, H. L. Prichard, R. D. Butler, B. Klitzman and M. H. Schoenfisch, Biomaterials, 2005, 26, 6984 CrossRef CAS.
- B. J. Nablo and M. H. Schoenfisch, Biomaterials, 2005, 26, 4405 CrossRef CAS.
- M. E. Robbins, B. K. Oh, E. D. Hopper and M. H. Schoenfisch, Chem. Mater., 2005, 17, 3288 CrossRef CAS.
- C. J. Brinker and G. Scherer, Sol–Gel Science: The Physics and Chemistry of Sol–Gel Processing, Academic Press, Boston, 1989 Search PubMed.
- J. H. Shin, S. M. Marxer and M. H. Schoenfisch, Anal. Chem., 2004, 76, 4543 CrossRef CAS.
- J. H. Shin, S. W. Weinman and M. H. Schoenfisch, Anal. Chem., 2005, 77, 3494 CrossRef CAS.
- B. K. Oh, M. E. Robbins, B. J. Nablo and M. H. Schoenfisch, Biosens. Bioelectron., 2005, 21, 749 CrossRef CAS.
- M. E. Robbins, E. D. Hopper and M. H. Schoenfisch, Langmuir, 2004, 20, 10296 CrossRef CAS.
- B. J. Nablo and M. H. Schoenfisch, Biomacromolecules, 2004, 5, 2034 CrossRef CAS.
- B. J. Nablo, A. R. Rothrock and M. H. Schoenfisch, Biomaterials, 2005, 26, 917 CrossRef CAS.
- S. M. Marxer, M. E. Robbins and M. H. Schoenfisch, Analyst, 2005, 130, 206 RSC.
- R. Gifford, M. M. Batchelor, Y. Lee, G. Gokulrangan, M. E. Meyerhoff and G. S. Wilson, J. Biomed. Mater. Res., 2005, 75A, 755 CrossRef CAS.
- K. S. Bohl and J. L. West, Biomaterials, 2000, 21, 2273 CrossRef CAS.
- M. C. Frost and M. E. Meyerhoff, J. Am. Chem. Soc., 2004, 126, 1348 CrossRef CAS.
- M. C. Frost and M. E. Meyerhoff, J. Biomed. Mater. Res., 2005, 72A, 409 CrossRef CAS.
- B. K. Oh and M. E. Meyerhoff, J. Am. Chem. Soc., 2003, 125, 9552 CrossRef CAS.
- B. K. Oh and M. E. Meyerhoff, Biomaterials, 2004, 25, 283 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
