A new class of light-fast oxonol dyes: organic-glass forming salts of oxonol anions and 4,4′-bipyridinium cations†
Yoshio
Inagaki
*,
Shin-ichi
Morishima
,
Koji
Wariishi
,
Naoki
Saito
and
Masaharu
Akiba
Synthetic Organic Chemistry Laboratories, Fuji Photo Film Co., Ltd., 210 Nakanuma, Minami-ashigara, Kanagawa 250-0193, Japan. E-mail: yoshio_inagaki@fujifilm.co.jp; Fax: +81 465 73 7920; Tel: +81 465 73 7052
First published on 13th December 2005
Abstract
Light-fastness of anionic oxonol dyes as measured in noncrystalline solid films was markedly improved by introducing 4,4′-bipyridiniums as counter cations.
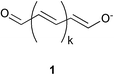
Oxonol dyes have an anionic chromophore based on a vinylogous carboxylate conjugate system (represented by a general formula 1), in which the terminal C–O− and C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O are often constituents of heterocycles.1 Despite such notable characteristics as the large extinction coefficients on the order of 105 dm−3 mol−1 cm−1, the narrow bandwidth, and the wide variation of absorption wavelengths over ultraviolet to near infrared, the vulnerability of oxonol dyes to light has limited the scope of application.2
O are often constituents of heterocycles.1 Despite such notable characteristics as the large extinction coefficients on the order of 105 dm−3 mol−1 cm−1, the narrow bandwidth, and the wide variation of absorption wavelengths over ultraviolet to near infrared, the vulnerability of oxonol dyes to light has limited the scope of application.2
In the course of development of new dyes for DVD-R (recordable digital versatile disk),3 which requires resistance to ambient light and a reading laser beam, we devised a new approach to enhance the light-fastness of oxonol dyes by causing the counter cation to quench the excited state of an oxonol anion, i.e. we introduced a new class of oxonol salts composed of an oxonol anion and an electron accepting bipyridinium.4
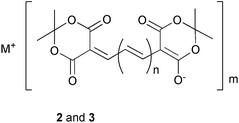
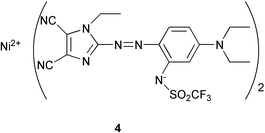
To accomplish this, we synthesized pentamethine (2a–2d) and heptamethine (3a, 3b) oxonol dyes based on Meldrum's acid with a variation of the counter cations as listed in Table 1.‡ As a standard for light-fastness, we employed a nickel-azo dye 4, which has actually been used in commercial DVD-Rs with practically sufficient light-fastness.5
| Compound | m | n | M+ |
|---|---|---|---|
| 2a | 1 | 2 | Et3NH+ |
| 2b | 1 | 2 | Me4N+ |
| 2c | 2 | 2 | N,N′-diphenyl-4,4′-bipyridinium2+ |
| 2d | 2 | 2 | N,N′-di(1-naphthyl)-4,4′-bipyridinium2+ |
| 3a | 1 | 3 | Et3NH+ |
| 3b | 2 | 3 | N,N′-diphenyl-4,4′-bipyridinium2+ |
These dyes were spin-coated onto glass plates using 2,2,3,3-tetrafluoro-1-propanol as a solvent to form noncrystalline-solid-dye films. They were colored, transparent, uniform, but dark when placed between crossed polarizers. These characteristics are typical of amorphous solids or so-called organic glasses. These films, supported on glass plates, were irradiated with a 170000-lux xenon lamp in a merry-go-round apparatus under air-cooling. The absorption spectra were intermittently monitored.
Fig. 1 shows the change of the normalized absorbance of dye films measured at their respective absorption maxima. The triethylammonium salts 2a and 3a faded rapidly. In striking contrast, the oxonol dyes with bipyridinium counter cations, 2c, 2d, and 3b, showed enhanced light-resistance. It is noteworthy that these oxonol dyes with bipyridinium cations exhibited light-fastness even comparable to the nickel-azo dye 4, because the heavy metal-free, light-fast, and soluble dye has been rather exceptional.
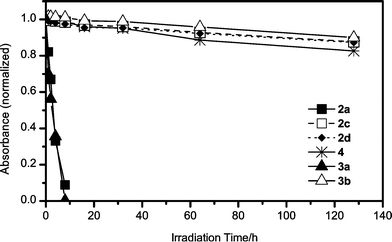 | ||
| Fig. 1 Changes in absorbance of dye films at their respective absorption maxima under irradiation with a 170000-lux xenon lamp. Absorption maxima of the films made of 2a, 2c, 2d, 3a, 3b, and 4 were 573, 569.5, 570, 685, 674, and 592 nm, respectively. | ||
In order to obtain an insight into the stabilizing mechanism, we measured fluorescence spectra. The solid film of 2a was fluorescent, but that of 2c was not (Fig. 2). A chloroform solution of 2b was strongly fluorescent. By contrast, a chloroform solution of 2c was only faintly fluorescent. These observations suggest that the counter cation quenched the singlet-excited state of the oxonol anion. Interestingly, in such polar solvents as methanol or acetonitrile, dyes 2b and 2c were strongly fluorescent and showed almost the same fluorescence spectra, probably because polar solvents favor separation of the ion pairs.6 In chloroform, fluorescence quenching seems to be facilitated by the short contact between the oxonol anion and the counter cation in the ion pair. Therefore, the quenching in chloroform was probably much faster than a diffusion-controlled rate due to the nature of the intra-ion pair.
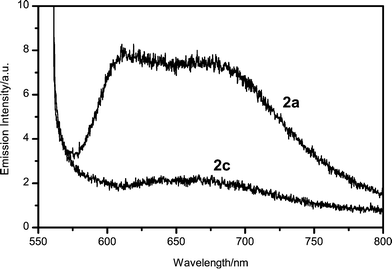 | ||
| Fig. 2 Fluorescence spectra of solid films made of 2a and 2c. Excitation wavelength is 550 nm. | ||
In chloroform, the intensity of the fluorescence of oxonol dye 2b declined with the addition of N,N′-diphenyl-4,4′-bipyridinium dichloride 5. A Stern–Volmer plot showed a straight line, which indicates that the bipyridinium cation quenched the singlet excited state of the oxonol anion. The Stern–Volmer factor (kqτ) was calculated as 4.3 × 105 mol−1 dm3. Assuming that the quenching rate constant remains at least two orders of magnitude larger than the diffusion controlled rate constant (1012 mol−1 dm3 s−1) in a contact ion pair, we estimate the lifetime of the emission (τ) to be of nano-second order (430 ns at the most), which is a reasonable value for the lifetime of a singlet-excited state.
These observations suggest that the oxidative quenching mechanism is responsible for the light-fastness of the oxonol salts: (1) an oxonol anion absorbs light, which results in an excited singlet state; (2) an electron-deficient counter cation accommodates one electron from the excited oxonol anion; and (3) an electron is transferred from the reduced counter cation to the oxidized oxonol to regenerate the oxonol salt in the ground state.
The oxidation and reduction potentials of the oxonol anion were 0.57 and −1.17 V vs. SCE (saturated calomel electrode), respectively, for 2a and 0.35 and −1.07 V vs. SCE, respectively, for 3a. The first reduction potential of the bipyridinium dication in 5 was −0.33 V, which corresponds approximately to the average of the reduction and oxidation potentials of the oxonol anions. Hence the free energy change for this scheme of electron flow is negative and consistent with the efficient quenching.
In conclusion, we have prepared a new class of light-fast oxonol dyes (salts of oxonol anions and 4,4′-bipyridinium cations). The light-fastness of the noncrystalline films made of the salts is much higher than that without bipyridinium cations and is even comparable to that of nickel-azo dye 4. In view of the usefulness of organic-glass or amorphous-solid forming dyes in such photonic devices as photovoltaic cells, organic light emitting diodes, and optical recording discs, the present finding of heavy-metal free and organic-glass forming oxonol dyes with enhanced light-resistance will expand the range of potential applications for oxonol dyes.
Notes and references
-
F. M. Hamer, The Cyanine Dyes and Related Compounds, Chemistry of Heterocyclic Compounds Vol. 18, Wiley Interscience Publishers, New York, London, 1964, ch. 13, pp. 463–488 Search PubMed
.
-
H. Zollinger, Color Chemistry: syntheses, properties, and applications of organic dyes and pigments, VCH, Weinheim, New York, Basel, Cambridge, 2nd revised edn, 1991, p. 362 Search PubMed
.
- H. Kubo, M. Shibata, H. Hashimoto, S.-i. Morishima, M. Akiba and Y. Inagaki, Jpn. J. Appl. Phys., 2004, 43(7B), 5038–5039 CrossRef CAS
.
- The following two works were useful for us to conceive this idea:
(a) S.-i. Morishima, K. Wariishi, Y. Inagaki, M. Shibata, T. Ishida and H. Kubo, Jpn. J. Appl. Phys., 1999, 38, 1634 CrossRef CAS
; (b) A. S. Tatikolov, L. A. Shevedova, Kh. S. Dzhulibekov, Zh. A. Krasnaya, A. L. Sigan and V. A. Kuz'min, Russ. Chem. Bull., 1995, 44, 851 CrossRef
.
-
(a) Y. Suzuki, M. Horie, Y. Okamoto, Y. Kurose and S. Maeda, Jpn. J. Appl. Phys., 1998, 37, 2084 CrossRef CAS
; (b) the synthetic procedure for 4 is disclosed in Jpn. Pat., Kokai Tokkyo Koho, 1999, H11-166125.
- Cation-anion dyes form ion pairs in weekly polar solvents, but dissociate in polar solvents: A. S. Tatikolov, Kh. S. Dzhulibekov, Zh. A. Krasnaya, E. V. Grechkina, V. I. Avdeeva and V. A. Kuz'min, Bull. Russ. Acad. Sci., Div. Chem. Sci., 1992, 41, 1985 Search PubMed
.
- A value in acetonitrile reported for a salt made of the oxonol anion of 2a and a cyanine cation4b.
Footnotes |
| † Electronic supplementary information (ESI) available: details of experimental procedure including syntheses, redox potentials, absorption spectra and a Stern–Volmer plot. See DOI: 10.1039/b516043j |
| ‡ Preparation of oxonol salts. 2c: triethyl amine (33.7 cm3, 0.24 mol) was added to a mixture of Meldrum's acid (24.2 g, 0.17 mol), 1,7-diphenyl-1,7-diazahepta-1,3,5-triene hydrochloride (19.8 g, 0.07 mol) and 50 cm3 of methanol and stirred for 3 h at rt. Water (300 cm3) and conc. HCl (30 cm3) were added to the mixture. The resulting violet crystals were collected by filtration, rinsed with ethyl acetate, and dried to give the free oxonol dye (15.6 g, 63.6%). N,N′-Diphenyl-4,4′-bipyridinium dichloride (0.23 g, 0.6 mmol) was added to a solution of the free oxonol dye (0.35 g, 1.0 mmol) in 10 cm3 of methanol. The resulting precipitates were collected by filtration, rinsed with methanol, and dried to give oxonol salt, 2c, as a green powder almost quantitatively (Found: C, 66.5; H, 5.1; N, 2.75. Calc. for C56H52N2O16: C, 66.7; H, 5.2; N, 2.8%); mp 201–202 °C (decomp.); m/z (+)310, (−)349; λmax(DMF)/nm 558.3 (ε/dm3 mol−1 cm−1 1.83 × 105); δH(400 MHz; DMSO-d6, Me4Si) 1.56 (24H, s), 7.17 (4H, t, J 13), 7.53 (2H, t, J 12.8), 7.68 (4H, d, J 13.6), 7.82 (6H, br s), 7.98 (4H, br s), 9.05 (4H, br s), 9.70 (4H, br s). Using N,N′-di(1-naphthyl)-4,4′-bipyridinium dibromide as a cation source, we obtained 2d as green crystals (Found: C, 68.0; H, 5.15; N, 2.5. Calc. for C64H56N2O16·H2O: C, 68.2; H, 5.2; N, 2.5%); mp 201.5–203 °C (decomp.); m/z (+)410, (−)349; λmax(DMF)/nm 558.3 (ε/dm3 mol−1 cm−1 1.90 × 105); δH(400 MHz, DMSO-d6, Me4Si) 1.57 (24H, s), 7.18 (4H, t, J 13), 7.53 (2H, t, J 12.6), 7.55 (2H, s), 7.69 (4H, d, J 13.6), 7.8 (4H, m), 7.90 (2H, br s), 8.14 (2H, br s), 8.30 (2H, d), 8.43 (2H, br s), 9.20 (4H, br s), 9.81 (4H, br s). Using Me4NBr as a cation source, we obtained 2b (Found: C, 51.5; H, 7.3; N, 3.0. Calc. for C21H29NO8·3.5H2O: C, 51.8; H, 7.7; N, 2.9%); mp 199.2–200 °C (decomp.); m/z (+)74, (−)349; λmax(DMF)/nm 558.3 (ε/dm3 mol−1 cm−1 1.88 × 105); δH(400 MHz, DMSO-d6, Me4Si) 1.57 (12H, s), 3.14 (12H, s), 7.17 (2H, t, J 13.2), 7.53 (1H, t, J 12.8), 7.67 (2H, d, J 13.6). Using 1,9-diphenyl-1,9-diazanona-1,3,5,7-tetraene hydrochloride, we obtained 3b as a powdery solid with a golden luster; mp 178–179 °C (decomp.); m/z (+)310, (−)375; λmax(NMP)/nm 664.5 (ε/dm3 mol−1 cm−1 2.38 × 105); δH(400 MHz, DMSO-d6, Me4Si), 1.57 (24H, s), 6.29 (2H, t, J 12.4), 7.18 (4H, t, J 13.2), 7.32 (4H, t, J 12.8), 7.57 (4H, d, J 13.6), 7.82 (6H, br s), 7.97 (4H, br s), 9.06 (4H, br s), 9.70 (4H, br s). |
| This journal is © The Royal Society of Chemistry 2006 |