Photoswitching of ferroelectric liquid crystals using photochromic dopants
Robert P.
Lemieux
Chemistry Department, Queen’s University, Kingston, Ontario, Canada K7L 3N6. E-mail: lemieux@chem.queensu.ca
First published on 10th October 2005
Abstract
This review focuses on the development of photochromic dopants designed to trigger the switching of ferroelectric liquid crystals via photomodulation of the spontaneous polarization (PS). The photomodulation of PS has been achieved by reducing the polar order of a FLC host via the change in shape of azobenzene dopants upon trans–cis photoisomerization, and via the loss of conformational flexibility of dithienylethene dopants upon photocyclization. It has also been achieved by increasing the polarization power of chiral thioindigo dopants in achiral SmC hosts via a photoinduced change in transverse dipole moment. A photoinversion of the sign of PS has been achieved using an “ambidextrous” thioindigo dopant with two competing chiral side-chains that induce polarizations of opposite handedness.
 Bob Lemieux | Bob Lemieux was born in Montréal, Québec in 1962. He received his BA from Colgate University in 1984 and his PhD in organic chemistry from the University of Illinois at Urbana–Champaign in 1989 under the direction of Peter Beak. He worked as a postdoctoral research associate at the University of Illinois under the direction of Gary Schuster from 1989 to 1992 and then joined the faculty at Queen's University, where he is presently Professor of Chemistry and Associate Head. His research interests include the design of new materials for the induction and photomodulation of chiral bulk properties in liquid crystal phases, and the study of non-covalent interactions and self-assembly in liquid crystals and other soft materials. |
Introduction
Many recent advances in information technology have arisen from the development of molecular switches that can modulate the bulk properties of a wide range of materials, including thermotropic liquid crystals.1 The latter are unique soft materials which have revolutionized the display industry, and hold tremendous potential in a wide range of advanced technologies such as telecommunication, information storage and sensors. By virtue of their fluid nature, liquid crystals are easily processed into uniform thin films, yet they exhibit the optical properties of crystalline materials such as the ability to rotate plane-polarized light due to birefringence. These ordered fluids can respond to one or more of a wide range of external stimuli including electric and magnetic fields, heat, mechanical force and light. Furthermore, liquid crystals are sensitive to perturbations exerted by dopants, which normally result in bulk property changes that may be useful in the development of new switching mechanisms.The most successful commercial application of liquid crystals remains the ubiquitous liquid crystal display, in which the molecular orientation of a liquid crystal film is driven between two different states using a weak electric field, thus producing an ON/OFF light shutter between crossed polarized filters.2 Most LCD applications currently on the market use multicomponent mixtures of uniaxial nematic liquid crystals and rely on the dielectric anisotropy of the liquid crystal phase to produce the required electro-optical effect. However, a significant proportion of new liquid crystalline materials being developed for the next generation of display applications as well as emerging non-display applications are chiral smectic liquid crystals,3 most notably ferroelectric smectic C* liquid crystals. FLC materials are currently used in commercial high-resolution reflective liquid-crystal-on-silicon (LCOS) microdisplays,4 and have promising potential applications in nonlinear optics,5 chiral sensing,6 and photonics devices.7–9 The SmC* phase is a biaxial liquid crystal phase with diffuse layer ordering and a uniform tilt θ of the molecular director n within each layer; in the absence of external alignment constraints, the SmC* phase forms a helical structure in which the director n precesses about the layer normal z.
Another chiral bulk property of the SmC* phase is a spontaneous electric polarization PS oriented along the C2 symmetry axis (polar axis) of each smectic layer.10 The spontaneous polarization originates in each chiral molecule forming the SmC* phase from a conformational bias of transverse dipoles to orient in one direction along the polar axis due to steric coupling of polar functional groups to the stereogenic center. The magnitude of PS is a function of the structure and proportion of the SmC* chiral component(s), and it can be left-handed (negative) or right-handed (positive) depending on the absolute configuration of the stereogenic center.11,12 The helical form of the SmC* phase is not ferroelectric as the polarization vectors add up to zero over one helical pitch. However, Clark and Lagerwall showed that the helical SmC* phase unwinds between rubbed polyimide-coated glass slides with a spacing on the order of the pitch (typically 1–5 µm) to give a surface-stabilized ferroelectric liquid crystal (SSFLC) with a net spontaneous polarization perpendicular to the plane of the glass slides (Fig. 1).13 A SSFLC can be switched from one tilt orientation to the other on a microsecond time scale by coupling the polarization to an electric field E (Goldstone-mode switching) to produce a bistable ON/OFF light shutter between crossed polarized filters. The performance characteristics of SSFLC devices, including the electro-optical switching time and photoswitching threshold (vide infra), depend in part on the magnitude of PS induced by the chiral component(s) of the SmC* phase.
 | ||
| Fig. 1 Goldstone-mode switching of a SSFLC via electric field inversion. The vectors z (layer normal) and n (molecular director) are in the plane of the page and form a tilt angle θ. The polar C2 axis is coincident with the PS vector and is normal to the plane of the page. | ||
A growing interest in liquid crystal devices for photonics and telecom applications has led to studies of photoswitching mechanisms that enable the inscription of images, diffraction gratings and waveguides on SSFLC films. Photoswitching of a SSFLC can be achieved extrinsically using a photoconductive or photodiode layer,7,14 or intrinsically by modulating the magnitude and/or sign of spontaneous polarization via the reversible photoisomerization of a photochromic dopant (Fig. 2). This review focuses on two different aspects of intrinsic SSFLC photoswitching: (i) the photomechanical effect using azobenzene and dithienylethene dopants, and (ii) transverse dipole photomodulation using chiral thioindigo dopants.
 | ||
| Fig. 2 Photoswitching of a SSFLC film by (a) photomodulation of PS below a coercive force threshold EC (top) and (b) photoinduced sign inversion of PS (bottom). In both cases, a dc electric field is maintained across the SSFLC film. | ||
Photomechanical effect
One approach to photomodulate the magnitude of PS is based on the change in molecular shape of a photochromic dopant that destabilizes the SmC* phase of a liquid crystal host. This so-called photomechanical effect causes a shift of the PSvs. temperature profile by lowering the Curie point, i.e., the upper transition temperature to a non-ferroelectric phase. At constant temperature, the photomechanical effect results in a decrease in polarization, or a complete loss of ferroelectric properties due to an isothermal phase transition. Ikeda et al. reported the first example of a photomechanical effect in a FLC host viatrans–cis photoisomerization of the 4,4′-disubstituted azobenzene dopant 1.15,16 Irradiation of a 2 µm SSFLC film consisting of a 3 mol% mixture of 1 in the liquid crystal host 2 at 360 nm causes a destabilization of the SmC* phase due to the change in shape of the azobenzene chromophore from rod-like (trans) to bent (cis), and a lowering of the coercive force EC required to switch the SSFLC. Ikeda et al. showed that photoswitching of the azobenzene-doped SSFLC can be achieved by setting the applied field E between EC thresholds corresponding to the trans-state and photostationary cis-state. According to time-resolved measurements, photoswitching of the azobenzene-doped SSFLC mixture is achieved in ca. 0.5 ms with a 10 ns irradiating pulse at 355 nm, and can be reversed viacis–trans photoisomerization at >450 nm.16 Many other examples of azobenzene-driven polarization photomodulation have been reported over the past decade, including systems combining chiral or achiral azobenzene dopants in FLC hosts,17–20 chiral azobenzene dopants in achiral SmC hosts,21,22 FLC side-chain copolymers with azobenzene units,23,24 and azobenzene FLCs.25SSFLC photoswitching via sign inversion of PS was demonstrated by Komitov and Ichimura with a 5 wt% mixture of the achiral azobenzene dopant 3 in a FLC host that undergoes a sign inversion of PS with temperature (4).20 In this case, photoswitching is achieved by setting the temperature of the azobenzene-doped SSFLC between inversion temperatures corresponding to the trans- and cis-photostationary states; upon irradiation, the photomechanical effect causes a shift in the PSvs. temperature profile which results in an isothermal sign inversion of PS.
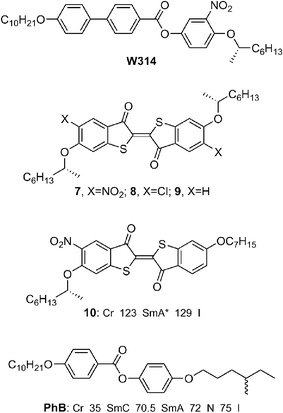
One inherent problem with azobenzene-driven photoswitches is a lack of photochromic bistability due to thermal reversion of the azobenzene cis-isomer to the more stable trans-isomer, which may limit their usefulness in some optical data storage applications. To address this problem in SSFLC photoswitches, we developed achiral dithienylethene dopants such as 5 which photomodulate the polarization of a FLC host mixture via a photomechanical effect.26,27 These compounds undergo a reversible conrotatory photocyclization reaction, as shown in Scheme 1, in which both photoisomers are thermally stable;28 the uncyclized form is colorless whereas the cyclized form absorbs strongly in the visible. Compound 5 was doped in a FLC host consisting of a 10 mol% mixture of the Displaytech dopant MDW950 in the achiral phenylpyrimidine liquid crystal PhP. Despite its relatively high aspect ratio, the uncyclized dopant 5 causes a significant destabilization of the FLC host, as evidenced by a shift in Curie point of 19 K in a 3 mol% mixture. Photocyclization of 5 at 313 nm causes the PSvs. temperature profiles to shift to lower temperatures (Fig. 3), the effect being more pronounced at the higher mole fraction, which is consistent with a photomechanical destabilization of the SmC* phase.
 | ||
| Fig. 3 Spontaneous polarization PSvs. temperature for the FLC mixture MDW950/PhP with no photochromic dopant (triangles), and doped with 5 at 1 mol% (circles) and 3 mol% (squares), before and after irradiation at 313 nm (open and filled symbols, respectively). | ||
 | ||
| Scheme 1 | ||
The high polarization state is restored by irradiation with visible light at >455 nm, and the photoswitching cycle can be repeated numerous times without any sign of photodegradation. Furthermore, the low polarization state remains stable in the dark for several days, thus proving that the doped SSFLC mixture is photochromically bistable. Interestingly, molecular modelling shows that, unlike 4,4′-disubstituted azobenzene dopants, the photocyclization of dithienylethene compounds such as 5 results in a relatively modest changes in molecular shape. As shown in Fig. 4, the AM1 model of 5 in its most extended form has an unfavorable bow-like shape that should disrupt the lamellar ordering of the FLC host, and which remains more or less the same upon photocyclization to 6. It is possible, in this case, that the photomechanical effect arises from a difference in conformational degrees of freedom of the uncyclized and cyclized isomers. The uncyclized isomer 5 is conformationally more flexible by virtue of rotation about the thienylethene single bonds, and should therefore be more adaptable to the diffuse lamellar ordering imposed by the SmC* phase. However, the dopant loses its conformational flexibility upon photocyclization, effectively “locking in” the unfavorable bow-like shape, which should cause a greater destabilization of the SmC* phase.
 | ||
| Fig. 4 Space-filling AM1 models of 5 (top) and its photocyclized isomer 6 (bottom). | ||
Transverse dipole photomodulation
The modulation of PSvia a photomechanical effect relies on a decrease in polar order of the FLC host and does not require the photochromic dopant to be chiral. An alternative approach to the photomechanical effect is to combine a photochromic chiral dopant with an achiral SmC host and modulate the induced polarization via a photodriven change in the polarization power of the chiral dopant. The polarization power δp is a measure of the propensity of a chiral dopant to induce a spontaneous polarization in a SmC liquid crystal host,29 which depends on the degree of steric coupling between the stereogenic center and any functional group with a dipole moment transverse to the molecular long axis (stereo-polar coupling). For instance, Walba showed that FLCs containing a chiral o-nitro-2-octyloxyphenyl unit, e.g., W314, exhibit high spontaneous polarizations due to steric coupling of the chiral alkoxy group to the polar o-nitrophenyl group, which forces their transverse dipole moments in the same direction along the polar axis of the SmC* phase.30 According to the Boulder model, the stereo-polar unit of W314 can adopt two staggered conformations in which the coupled alkoxy/o-nitrophenyl dipoles are oriented along the polar axis (the third staggered conformation orients the dipoles in the tilt plane and cannot contribute to PS), as shown in Fig. 5.11 These two conformations are nonequivalent, with the anti conformation being favored over the gauche conformation, which corresponds to a negative polarization in accordance with experimental observations.30 | ||
| Fig. 5 AM1-minimized conformations of the (S)-2-octyloxy side-chain of W314 in the SmC* phase according to the Boulder model, and as Newman projections about the C2–C3 bond. The sign of PS conforms to the physics convention and points from negative to positive. | ||
In designing a chiral dopant with photovariable polarization power, we sought a system that undergoes photoisomerization between isomers with different transverse dipole moments, and one that can be coupled to a chiral side-chain to convert the change in transverse dipole moment into a change in induced polarization. The chiral thioindigos 7 and 8 represent the first generation of such dopants.31,32 The thioindigo chromophore undergoes trans–cis photoisomerization in the visible range of the spectrum, and the absorption maxima of the trans- and cis-isomers are well resolved, as shown in Fig. 6. Molecular modelling suggests that dopants such as 7 and 8 maintain a rod-like shape in both isomeric forms, thus minimizing the photomechanical destabilization of an achiral SmC host. Positioning of the nitro and chloro substituents ortho to the (R)-2-octyloxy side-chains, as in W314, ensures that the thioindigo core is strongly coupled to the stereogenic centers, which is a key requirement for the photoinduced change in transverse dipole moment of the core to produce a modulation in PS. Indeed, initial work showed that photoisomerization of the uncoupled thioindigo dopant 9 in the racemic host PhB has virtually no effect on the induced polarization.33
 | ||
Fig. 6 Visible absorption spectrum of a 10−4 M solution of 7 in benzene in the trans form (—), and in the cis form after irradiation at 514 nm (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ). ). | ||
Doping the racemic host PhB with either 7 or 8 induces a ferroelectric SmC* phase with a positive sign of PS, which is consistent with that predicted by the Boulder model. The solubility of the dinitro derivative 7 in PhB is very low, with a saturation point of ca. 3 mol%. By contrast, the dichloro derivative 8 dissolves readily in PhB to form homogeneous solutions up to 10 mol% according to texture analysis by polarized microscopy. However, subsequent deuterium NMR studies on solutions of deuterated 8 in PhB revealed a high degree of microphase separation and partitioning of the dopant between smectic and isotropic domains at dopant mole fractions as low as 4 mol%.34 This lack of compatibility with the SmC host is thought to be due to an unfavorable packing requirement of the two branched side-chains in a symmetrical configuration. A more recent deuterium NMR study has shown that unsymmetrical thioindigo dopants with a single branched side-chain are highly miscible with PhB—one particular derivative (10) even forms a SmA* liquid crystal phase.35
As shown in Fig. 7a, the spontaneous polarization of a 2.6 mol% mixture of 7 in PhB nearly doubles upon irradiation at 514 nm, which is consistent with an increase in transverse dipole moment of the chiral thioindigo dopant upon trans–cis photoisomerization.31 Extrapolation of the two plots to PS = 0 shows that PS photomodulation is achieved without an appreciable shift in the Curie point, i.e., without concomitant destabilization of the SmC* phase. At a comparable mole fraction, the trans–cis photoisomerization of an azobenzene-containing FLC causes a 15 K drop in the Curie point.23 Similar experiments were carried out with mixtures of 8 in PhB over a wider mole fraction range. As shown in Fig. 7b, irradiation of a 1.9 mol% mixture at 532 nm results in a 4-fold increase in PS, but the degree of PS photomodulation decreases with increasing dopant mole fraction, to less than two-fold at 10 mol%.32 This effect has also been observed with unsymmetrical thioindigo dopants in PhB (vide infra), and is consistent with a suppression of trans–cis photoisomerization due to aggregate formation. An analysis by UV–vis spectroscopy showed that the degree of trans–cis photoisomerization of 10 as a 1 mol% solution in PhB (ca. 0.02 M) is ca. 90% less than as a dilute 10−4 M solution in benzene, and further decreases with increasing mole fraction in PhB.35
 | ||
| Fig. 7 (a) Spontaneous polarization PS vs. temperature for a 2.6 mol% mixture of 7 in PhB in the dark (filled circles) and under irradiation at 514 nm (open circles). (b) Spontaneous polarization PSvs. dopant mole fraction xd measured at 10 K below the Curie point for mixtures of 8 in PhB in the dark (black columns) and under irradiation at 532 nm (white columns). | ||
The sign of PS can be photoinverted with a chiral thioindigo dopant such as 7 or 8 when used in conjunction with a photoinert chiral dopant that induces a polarization of opposite sign.36 For example, we formulated a FLC mixture consisting of the host PhB, the chiral thioindigo dopant 8, which induces a positive polarization (in black), and the chiral diester 11, which induces a negative polarization (in grey). The dopant mole fractions were adjusted to 3.0 and 1.3 mol%, respectively, to give a net negative polarization in the dark (i.e., the negative polarization induced by 11 is predominant when 8 is in the trans form). Upon irradiation at 532 nm, the positive polarization induced by 8 increases by a factor of ca. 2.5 while the negative polarization induced by 11 remains unchanged, which results in a net inversion in the sign of PS, as depicted schematically in Fig. 8. With a dc field applied across the SSFLC film, photoinversion of the sign of PS results in Goldstone-mode switching.
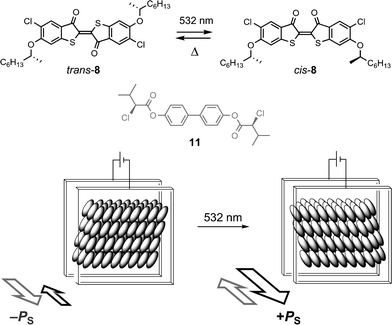 | ||
| Fig. 8 Photoswitching of a FLC mixture with two chiral dopants inducing polarizations of opposite signs: the thioindigo 8 (3 mol%) and the diester 11 (1.3 mol%) in the host PhB. | ||
This approach to polarization photoinversion is based on a competition between two chiral dopants with polarization powers of opposite sign, or handedness. More recently, we extended the design of unsymmetrical thioindigos such as 10 to produce a new chiral thioindigo dopant 12 with competing stereo-polar units of opposite handedness, i.e., an “ambidextrous” dopant similar conceptually to a switch hitter in baseball.37,38 A similar concept has been used by Green et al. in polyisocyanates with different chiral units competing to control the helical sense of the polymer chain.39 In the ambidextrous dopant 12, the (R)-2-octyloxy side-chain and the thioindigo core are strongly coupled via the nitro substituent and form one stereo-polar unit that induces a positive polarization. Conversely, the Displaytech (R,R)-2,3-difluorooctyloxy side-chain, which induces a negative polarization, is decoupled from the thioindigo core and behaves more or less independently of the latter. With this design, the increase in transverse dipole moment of the thioindigo chromophore upon trans–cis photoisomerization raises the polarization power of the Displaytech side-chain above that of the coupled thioindigo core and inverts the net sign of polarization.
The polarization power of 12 in PhB at 5 K below the Curie point is −309 nC cm−2, which is approximately the sum of δp values reported for dopants with either the Displaytech side-chain or the coupled thioindigo core.35,40 This result confirmed that the latter predominates when the thioindigo core is in the trans configuration. As shown in Fig. 9, irradiation of a 4 mol% mixture of 12 in PhB at 510 nm causes a decrease in polarization, but no sign inversion, which is consistent with an increase in the polarization power of the coupled thioindigo core upon trans–cis photoisomerization. Lowering the dopant mole fraction to 1 mol% leads to a 2.8-fold increase in trans–cis photoconversion, as observed with the analogous dopant 10, and in the inversion of the sign of polarization. As with other chiral thioindigo dopants, the polarization photoinversion is achieved without concomitant destabilization of the SmC* phase. The occurrence of polarization photoinversion was confirmed by a photoswitching experiment performed with a 1 mol% mixture of 12 in the Displaytech mixture MX6120 (a low viscosity SmC mixture of phenyl benzoate and phenylpyrimidine components) as a well aligned 4 µm SSFLC film positioned between crossed polarized filters. The intensity of a 665 nm diode laser reading beam passing through the SSFLC assembly is measured as a function of irradiation conditions using a photodetector, which is first calibrated by applying a square wave ac field (±10 V) to produce an electrically driven Goldstone-mode switching (Fig. 10). The voltage across the film is then switched to a 10 V dc field and the film is irradiated at 510 nm, which causes a change in transmittance consistent with Goldstone-mode switching. Turning off the 510 nm beam results in thermal relaxation of 12 to the trans-isomer, which restores the negative polarization and causes the SSFLC to switch back.
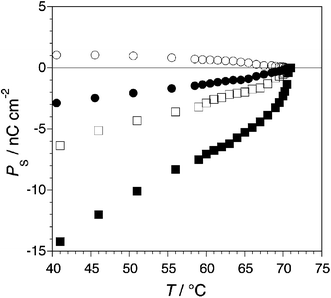 | ||
| Fig. 9 Spontaneous polarization PSvs. temperature for 4 mol% (squares) and 1 mol% (circles) mixtures of 12 in PhB, in the dark (filled symbols) and under irradiation at 510 nm (open symbols). | ||
 | ||
| Fig. 10 Change in intensity of a 665 nm beam passing through a 1 mol% mixture of 12 in MX6120 as a SSFLC film between crossed polarized filters upon irradiation at 510 nm. On the left side, a square-wave ac voltage is applied across the film; on the right side, a dc voltage of 10 V is applied across the film. The measurements were taken at 10 K below the Curie point. | ||
Summary
This review has highlighted how one can photochemically modulate the spontaneous polarization of a ferroelectric liquid crystal either by reducing the polar order of the SmC* phase via a photoinduced change in shape or rigidity of a chiral or achiral dopant, or by modulating the polarization power of a chiral dopant via a photoinduced change in transverse dipole moment. In the latter case, photoswitching of a surface-stabilized FLC electro-optical device can be achieved via inversion of the sign of polarization by controlling, at the molecular level, the competition between chiral structural units of opposite handedness. Such control is made possible by our growing understanding of the relationship between the molecular structure of chiral dopants and the magnitude of the spontaneous polarization they induce.References
- Molecular Switches, ed. B. L. Feringa, Wiley-VCH, Weinheim, 2001 Search PubMed.
- I. C. Sage, in Handbook of Liquid Crystals, ed. D. Demus, J. W. Goodby, G. W. Gray, H.-W. Spiess and V. Vill, Wiley-VCH, Weinheim, 1998, vol. 1 Search PubMed.
- G. W. Gray and J. W. Goodby, Smectic Liquid Crystals, Leonard Hill, London, 1984 Search PubMed.
- (a) S. T. Lagerwall, Ferroelectric and Antiferroelectric Liquid Crystals, Wiley-VCH, Weinheim, 1999 Search PubMed; (b) S. T. Lagerwall, in Handbook of Liquid Crystals, ed. D. Demus, J. W. Goodby, G. W. Gray, H.-W. Spiess and V. Vill, Wiley-VCH, Weinheim, 1998, vol. 2B Search PubMed; (c) D. M. Walba, Science, 1995, 270, 250 CAS; (d) N. A. Clark and S. T. Lagerwall, in Ferroelectric Liquid Crystals: Principles, Properties and Applications, ed. J. W. Goodby, R. Blinc, N. A. Clark, S. T. Lagerwall, M. A. Osipov, S. A. Pikin, T. Sakurai, K. Yoshino and B. Zeks, Gordon and Breach, Philadelphia, 1991, pp 409–452 Search PubMed; (e) J. Dijon, in Liquid Crystals: Applications and Uses, ed. B. Bahadur, World Scientific, Singapore, 1990, vol. 1 Search PubMed.
- (a) D. M. Walba, L. Xiao, P. Keller, R. Shao, D. Link and N. A. Clark, Pure Appl. Chem., 1999, 71, 2117 CrossRef CAS; (b) D. M. Walba, D. J. Dyer, T. Sierra, P. L. Cobben, R. Shao and N. A. Clark, J. Am. Chem. Soc., 1996, 118, 1211 CrossRef CAS; (c) D. M. Walba, M. B. Ros, N. A. Clark, R. Shao, M. G. Robinson, J. Y. Liu, K. M. Johnson and D. Doroski, J. Am. Chem. Soc., 1991, 113, 5471 CrossRef CAS.
- D. M. Walba, D. J. Dyer, J. A. Rego, J. Niessink-Trotter, R. Shao and N. A. Clark, Ferroelectrics, 2004, 309, 121 CrossRef CAS.
- W. A. Crossland and T. D. Wilkinson, in Handbook of Liquid Crystals, ed. D. Demus, J. W. Goodby, G. W. Gray, H.-W. Spiess and V. Vill, Wiley-VCH, Weinheim, 1998, vol. 1 Search PubMed.
- T. Ikeda and A. Kanazawa, in Molecular Switches, ed. B. L. Feringa, Wiley-VCH, Weinheim, 2001 Search PubMed.
- R. P. Lemieux, Chem. Rec., 2004, 3, 288 Search PubMed.
- R. B. Meyer, L. Liebert, L. Strzelecki and P. Keller, J. Phys. Lett., 1975, 36, L69 Search PubMed.
- D. M. Walba, in Ferroelectric Liquid Crystals: A Unique State of Matter, ed. T. E. Mallouck, JAI Press, Ltd., Greenwich, CT, 1991 Search PubMed.
- (a) J. W. Goodby, E. Chin, T. M. Leslie, J. M. Geary and J. S. Patel, J. Am. Chem. Soc., 1986, 108, 4729 CrossRef CAS; (b) J. W. Goodby and E. Chin, J. Am. Chem. Soc., 1986, 108, 4736 CrossRef CAS.
- N. A. Clark and S. T. Lagerwall, Appl. Phys. Lett., 1980, 36, 899 CrossRef CAS.
- G. Moddel, in Spatial Light Modulator Technology: Materials, Devices, and Applications, ed. U. Efron, Marcel Dekker, New York, 1995, p. 287 Search PubMed.
- T. Ikeda, T. Sasaki and K. Ichimura, Nature, 1993, 361, 428 CrossRef CAS.
- T. Sasaki, T. Ikeda and K. Ichimura, J. Am. Chem. Soc., 1994, 116, 625 CrossRef CAS.
- H. G. Walton, H. J. Coles, D. Guillon and G. Poeti, Liq. Cryst., 1994, 17, 333 CrossRef CAS.
- T. Sasaki and T. Ikeda, J. Phys. Chem., 1995, 99, 13002, 13008, 13013 CrossRef CAS.
- A. Langhoff and F. Giesselmann, Ferroelectrics, 2000, 244, 283 CrossRef CAS.
- L. Komitov, O. Tsutsumi, C. Ruslim, T. Ikeda, K. Ichimura and K. Yoshino, J. Appl. Phys., 2001, 89, 7745 CrossRef CAS.
- M. Negishi, O. Tsutsumi, T. Ikeda, T. Hiyama, J. Kawamura, M. Aizawa and S. Takehara, Chem. Lett., 1996, 319 CAS.
- L. M. Blinov, M. V. Kozlovsky, K. Nakayama, M. Ozaki and K. Yoshino, Jpn. J. Appl. Phys., 1996, 35, 5405 CrossRef CAS.
- T. Öge and R. Zentel, Macromol. Chem. Phys., 1996, 197, 1805 CrossRef.
- J. G. Meier, J. Stumpe, B. Fischer, C. Thieme, T. M. Fischer, F. Kremer, T. Öge and R. Zentel, Polym. Adv. Technol., 1998, 9, 665 CrossRef CAS.
- G. Joly, A. Anakkar, M. Ismaili, P. Cluzeau, N. Isaert and H. T. Nguyen, Ferroelectrics, 2002, 277, 67 CrossRef CAS.
- K. E. Maly, M. D. Wand and R. P. Lemieux, J. Am. Chem. Soc., 2002, 124, 7898 CrossRef CAS.
- K. E. Maly, P. Zhang, M. D. Wand, E. Buncel and R. P. Lemieux, J. Mater. Chem., 2004, 14, 2806 RSC.
- M. Irie, Chem. Rev., 2000, 100, 1685 CrossRef CAS.
- K. Siemensmeyer and H. Stegemeyer, Chem. Phys. Lett., 1988, 148, 409 CrossRef CAS.
- D. M. Walba, M. B. Ros, N. A. Clark, R. Shao, K. J. Johnson, M. G. Robinson, J.-Y. Liu and D. Doroski, Mol. Cryst. Liq. Cryst., 1991, 198, 51 CAS.
- L. Dinescu and R. P. Lemieux, J. Am. Chem. Soc., 1997, 119.
- L. Dinescu, K. E. Maly and R. P. Lemieux, J. Mater. Chem., 1999, 9, 1679 RSC.
- L. Dinescu and R. P. Lemieux, Liq. Cryst., 1996, 20, 741 CAS.
- K. E. Maly, G. Wu and R. P. Lemieux, Liq. Cryst., 2001, 28, 457 CrossRef CAS.
- J. Z. Vlahakis and R. P. Lemieux, J. Mater. Chem., 2004, 14, 1486 RSC.
- L. Dinescu and R. P. Lemieux, Adv. Mater., 1999, 11, 42 CrossRef CAS.
- J. Z. Vlahakis, M. D. Wand and R. P. Lemieux, J. Am. Chem. Soc., 2003, 125, 6862 CrossRef CAS.
- J. Z. Vlahakis, M. D. Wand and R. P. Lemieux, Adv. Funct. Mater., 2004, 14, 637 CrossRef CAS.
- K. S. Cheon, J. V. Selinger and M. M. Green, Angew. Chem., Int. Ed., 2000, 39, 1482 CrossRef CAS.
- W. N. Thurmes, M. D. Wand, R. T. Vohra, K. M. More and D. M. Walba, Liq. Cryst., 1993, 14, 1061 CAS.
| This journal is © The Royal Society of Chemistry 2005 |