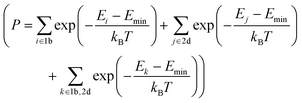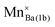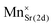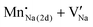Dual spectra band emissive Eu2+/Mn2+ co-activated alkaline earth phosphates for indoor plant growth novel phosphor converted-LEDs†
Young Jun
Yun
a,
Jin Kyu
Kim
b,
Ji Young
Ju
b,
Seul Ki
Choi
b,
Woon Ik
Park
c,
Jae Yong
Suh
d,
Ha-kyun
Jung
b,
Yongseon
Kim
 *e and
Sungho
Choi
*e and
Sungho
Choi
 *b
*b
aMaterials & Components Research Institute/Convergence Composite Materials Team, Korea Testing & Research Institute, 98 Gyoyukwon-ro, Gwacheon, Gyeonggi-do, Republic of Korea
bAdvanced Battery Materials Research Group, Korea Research Institute of Chemical Technology, 141 Gajeongro, Yuseong, Daejeon, Republic of Korea. E-mail: shochoi@krict.re.kr; Fax: +82-42-861-4151
cElectronic Convergence Materials Division, Korean Institute of Ceramic Engineering and Technology, 101 Soho-ro, Jinju, Gyeongsangnam-do, Republic of Korea
dDepartment of Physics, Michigan Technological University, Houghton, Michigan 49931, USA
eDepartment of Materials Science and Engineering, Inha University, Incheon, Republic of Korea. E-mail: ys.kim@inha.ac.kr
First published on 20th April 2017
Abstract
This paper reports designing a novel single composition blue/red color illuminating phosphor followed by fabricating “smart” agricultural/horticultural LED lighting. Color-tunable Eu2+/Mn2+ co-activated alkaline earth phosphates, Na(Sr,Ba)PO4 and Ca3Mg3(PO4)4, are considered, and the stable doping sites for the corresponding activators are identified by using first-principle DFT calculations. We can realize the designated color purity with stable thermal quenching preserved luminescence behavior is induced by the Eu2+ center positioned at different coordination states with intermixed Sr2+/Ba2+ sites in Na(Sr,Ba)PO4 hosts. Moreover, we demonstrate that the resultant LED lighting adopting the proposed novel phosphor composition stimulates the enhanced photosynthesis reaction for indoor hydroponics plants, such as oats and onions, which is superior to the narrow line emission band induced by the mixture of conventional red/green/blue LEDs. Thus, using the color-tunable single composition luminescent material may produce an innovative energy-efficient artificial lighting for indoor plant growth.
Introduction
Lighting sources are compulsory for the conventional indoor agriculture/horticulture industry and have mainly two functions during plant growth and development. First, light affects the overall growth rate of the plant (stem thickness, rooting, and branching) via the photosynthetic reaction. Second, light influences several developmental processes, such as seed germination and flowering. Thus, knowledge in the photoperiodic response of different greenhouse crops can help in scheduling flowering as well as reducing production time. Although natural sunlight is the cheapest source available, for horticulture it is not always attainable in sufficient quantities, particularly over the past years. Therefore, the use of artificial light has become very common for increasing production and quality.1,2Red- and blue-ray spectra may well drive the photosynthetic metabolism, and so it is quite reasonable that these band spectra, with the appropriate dose, would be particularly efficient when associated with autotrophic growth habits. Photosynthetically inefficient light qualities also impart important environmental information to a developing plant. For example, far-red light reverses the effects of phytochromes, leading to changes in gene expression, plant architecture, and reproductive responses, while the effects of green light oppose those directed by red and blue wavebands.3–5
Very recently, phosphor converted-white light emitting diodes (wLEDs) have prevailed across the IT industry and have gradually taken over the conventional solid-state lighting such as incandescent and fluorescent bulbs.6,7 Additionally, LED lighting used in modern agriculture and plant factories has developed rapidly in recent days as well. Different from the sensitivity of human eyes to light, the photosynthetic action spectrum of chlorophylls covers red and blue light. Thus, it is difficult to use traditional light sources for general lighting purposes in the field of plant lighting owing to the serious spectral mismatch between the emitted spectrum of lighting and plants.
Outdoor conditions are mimicked using various colors, temperatures and spectral outputs from the growth light and various lumen outputs for the lamps. Depending on the type of plant, the stage of cultivation (e.g., the germination and vegetative phase or the flowering/fruiting phase) and the photoperiod required by the plants, different ranges of the spectrum, luminous efficacy and color temperature are desirable to be applied for different plants and time periods. Compared to other types of growth lights, LEDs for indoor plants are attractive because they do not require ballasts and produce considerably less heat than incandescent lights. Further, plants under LEDs transpire less as a result of the reduction in heat, and thus the time between watering cycles is longer.8,9
Unfortunately, the present agricultural LED exhibits an illuminating spectrum with a very narrow line-shaped emission band induced by the assembly of individual red/green/blue LEDs. Thus, it is necessary to manipulate the resultant wavelength for the optimum photosynthetic-stimulating light dose via individually controlled multiple LEDs with a bulky and complicated electric apparatus to drive the overall lighting.
The most common method to achieve a customized color illumination from LEDs to date is to partially convert blue or ultraviolet light from the III–V semiconductor to a lower-energy emission by over-coating phosphors onto the LED chip. To fulfill the requirements of this method, the phosphors must have a high quantum efficiency, suitable color purity and stable thermal quenching/hydrolytic reliability.10–12 Although many studies have been performed to develop efficient phosphors for use in wLEDs, only limited compositions in the aluminate, orthosilicate, fluoride and nitrides compounds can be practically applied to wLEDs. Along with the emission color, quantum efficiency and thermal stability of the promising LED phosphors, a cost effective synthetic procedure is a big challenge for those phosphors that have failed to be commercialized.13
Various activator doped orthophosphates, AB(PO4) (A = alkali metal ion and B = alkali earth metal ion), have been studied widely for their potential application as luminescent materials. Most of all, Eu2+/3+ activated phosphates have been recommended as highly efficient phosphors for ultraviolet (UV) excitation.14–19 Since the phosphate compounds have various polymorphic crystal structures with host metal ions with different host metal ionic radii, the resultant phosphors exhibit different band emissions; e.g., orthorhombic KSrPO4:Eu2+ has a blue emission while Eu2+-doped orthorhombic NaCaPO4 has a broad green emission centered at 506 nm.15–17 H. J. Seo et al. reported the structural occupation with corresponding luminescence properties of trivalent Eu and Tb sites in Na(Sr,Ba)PO4.14,19 Additionally, various Eu2+ and Mn2+ codoped phosphors with blue and red emission bands have been extensively investigated.20–23 As a promising sensitizer for the Mn2+ ion, Eu2+ has been widely applied in many Mn2+-doped hosts, such as Ca9Y(PO4)7,21 Ca3Mg3(PO4)4,22 and Na2SrMg(PO4)2,23 phosphors.
Here, we propose a novel strategy to fabricate “smart” agricultural/horticultural LED lighting overcoated single composition blue/red emission phosphors, especially for Na(Sr0.5Ba0.5)PO4:Eu2+,Mn2+. There have been several studies on the white light phosphor-converted-LEDs for plant cultivation.24–27 Nevertheless, one critical issue lies in the limited luminescence results of the given phosphor due to the very low quantum efficiency. Additionally, empirical data from controlled experiments indicate that the resultant lighting for an enhanced photosynthesis reaction is insufficient. The Na(Sr0.5Ba0.5)PO4:Eu2+,Mn2+ phosphor converted LED lighting from our study offers a well-matched emission band with the customized absorption spectrum of the maximum photosynthetic reaction in indoor plants, such as oats. A key point in using two band emission phosphors is the achievement of a unique phosphor composition, a host compound with different activator species, that matches with the maximum photoperiodic response of different greenhouse crops. This illumination provides supplemental lighting to increase the rate of photosynthesis, especially during periods when the intensity of sunlight is low. Importantly, the phosphor-converting wavelength-tailored LEDs produce bright and long-lasting growth lights of the wavelengths overlapping with the multiple absorption peaks of the various plant's photochemical processes.
Experimental section
Synthesis of phosphors
Eu2+ and Mn2+ codoped Na(Sr0.5Ba0.5)PO4 phosphors (called NSBP) were synthesized by a solid-state reaction method. Na2CO3 (99%, High Purity Chemicals), SrCO3 (99.9%, High Purity Chemicals), BaCO3 (99%, High Purity Chemicals), (NH4)2HPO4 (99.99%, Sigma-Aldrich), Eu2O3 (99.99%, Rare Earth Co.), and MnCO3 (99.9%, High Purity Chemicals) were used without any purification. The materials were weighed and thoroughly ground with acetone. The mixture was first heated up to 600 °C and kept at this temperature for 1 h in an air atmosphere, then calcined at 1150 °C for 10 h in a reducing atmosphere of H2 (20%)/N2 (80%). Similarly, a series of Eu2+ and Mn2+ doped Ca3Mg3(PO4)4 phosphors (called CMP) was synthesized by a high temperature solid-state reaction, starting from a mixture containing CaCO3 (99.9%, High Purity Chemicals), MgCO3 (99.99%, High Purity Chemicals), (NH4)2HPO4 (99.99%, Sigma-Aldrich), Eu2O3 (99.99%, Rare Earth Co.), and MnCO3 (99.9%, High Purity Chemicals) in the given stoichiometric ratio. The powder reactants were mixed and ground thoroughly in an agate mortar. The mixture was first heated at 500 °C for 5 h in the furnace. Then, in order to obtain the final product, the above precursor was ground again and heated up to 1150 °C for 4 h in a slightly reducing atmosphere of H2 (5%)/N2 (95%).Materials characterization
The crystalline phase of the prepared phosphors was identified with a powder diffractometer (Rigaku Ultima IV Diffractometer) with a graphite-monochromator equipped with Cu Kα radiation operating at 40 kV and 40 mA. The excitation and emission spectra were measured using a photoluminescence (PL) system with an Xe lamp. The temperature dependent emission properties were measured using an optical fiber equipped on a self-made thermostatic chamber ranging from room temperature to 180 °C. The injection-current dependent EL measurements for the pc-LEDs were measured in an integrating sphere by a spectrometer at various applied voltages. For the LED chip fabrication, commercially available silicone encapsulants (EG6301 A and B, Dowhitech Co.) were used. The corresponding powder paste was mounted on the near-UV LED chip (purchased from SemiLEDs, λem = 395–400 nm, Pout = 12–15 mW).First-principles investigation of crystal structure
The crystal structure of Na(Sr0.5Ba0.5)PO4 was investigated by first-principles calculations. First, the site distribution of Sr2+ in the crystal was evaluated, and then the most stable doping site for Eu2+ and Mn2+ was examined, which was performed based on the energy calculation for crystal models with various locations of Sr2+ and Eu2+ or Mn2+. A (NaMePO4)8 supercell was used as the frame structure (Me = Ba2+, Sr2+), and the energy of each crystal model was calculated using density functional theory (DFT) based on the Perdew–Burke–Ernzerhof generalized gradient approximation with kjpaw-type pbesol pseudopotentials.28,29 QUANTUM-ESPRESSO30 code was used with 40 Ry of kinetic energy cutoff for wavefunctions and 10−3 Ry Bohr−1 of the convergence threshold on forces for ionic minimization. Spacing of k-points was 0.5 Å−1 for each lattice vector, and full relaxation of the atomic positions and lattice vectors was allowed during the calculation. The standard state chemical potential of oxygen was determined in a semi-empirical method, which is explained in detail in ref. 31 and 32.Evaluation for plant growth lightings
Oats and onions were initiated and grown for 30 days. Each study, consisting of one cultivar with one lamp type, was conducted in separate reach-in plant growth chambers. At harvest, plant height (main stem length) and number of nodes were recorded. Environmental conditions consisted of a whole 24 h-light (12 h-light/12 h-dark for onions) photoperiod with a matching thermoperiod of 20 ± 2 °C and a constant relative humidity of 65%. Atmospheric CO2 was maintained at 500 ppm and the average illuminance at 200 lx. Both the plant growth setup image of the thermo-hygrostat chamber and the in situ image of oats growing under LED illumination are appended in the ESI.†Result and discussion
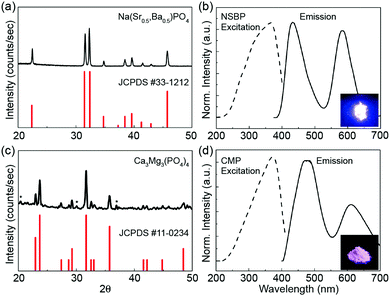
The X-ray diffraction (XRD) patterns and the corresponding excitation/emission spectra of NSBP and CMP are shown in Fig. 1. Note that the Eu2+/Mn2+ molar ratio is fixed in each composition, which means the tailored red/blue emission band position and relative intensity leads to cost-effective light sources that well stimulate the plant growth rate. To support the concentration quenching behavior of the given phosphor composition, both the excitation and emission spectra with different Eu2+/Mn2+ molar ratios are presented in Fig. S1 (ESI†). First, no traceable amounts of impurity phases were detected and all the reflections could be well indexed to a hexagonal olgite structure with the P3 space group. Thus, the as-prepared NSBP samples are single phase and the incorporated co-activators do not cause any significant change. For CMP, however, there might be some low intensity peaks (marked as *) that could have originated from the presence of Ca- or Mg-phosphate, but they were not taken into consideration for the luminescent behavior because neither of these impurities were active under the given photoexcitation. Even though acquiring the phase pure compound is stringent to guarantee the overall luminescence property, the resultant LED lighting adopting the dual-band emissive phosphor composition fruitfully stimulates the photosynthesis reaction for indoor plants (will be shown later). Moreover, the crystallographic coordination state and ionic radii of activators (Eu2+ and Mn2+) substituted for the host ion in a monoclinic structure CMP crystal lattice matched the previous work well.22The Eu2+ ion, as a typical and highly efficient activator with a strong, broad excitation band covering the emissions from near UV LED chips, was doped into the host lattice used for LEDs.15–18,20–24,27,33,34 Further, the Mn2+ ion, with a transition energy between the 4T1 and 6A1 levels, as a representative ion among transition metal ions, has a broadband emission with colors of green to deep red (from the weak field to the strong field interactions) depending on the crystal field strength.34–37
The overall excitation spectra show a broad absorption band within the 250–400 nm range; this is due to the transition from the 4f7 ground state of Eu2+ to the 4f65d1 excited state. We can obtain the specific blue emission band centered at 450 nm, which corresponds to the 4f–5d transition of Eu2+, and the six-fold coordinated Mn2+ induced broad orange/red emission band positioned at 600 nm. The Eu2+/Mn2+ system is one of the most abundant systems for emission color adjustment in phosphors.20–24 The degree of spectral overlap between the blue-region emission spectrum of Eu2+ and the excitation spectrum of Mn2+ is critical to generating an efficient energy transfer from Eu2+ to Mn2+, which produces tunable colors in an appropriate host compound. This study mainly focused on NSBP:Eu2+,Mn2+, which has a high output efficiency in pc-LEDs and excellent thermal quenching properties, which make it promising for application in plant growth lighting. A previous study reported by Wu et al. was helpful for understanding the energy transfer between Eu2+ and Mn2+ followed by the dual band emission behavior of the CMP:Eu2+,Mn2+ phosphor.22
It has been reported that NaBaPO4 belongs to the P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1 space group, in which Na+ ions occupy 1a sites and Ba2+ ions 1b sites, and 2d sites are occupied by 50% of Na+ and 50% of Ba2+ ions.38 However, the distribution of ions in NSBP has not yet been identified, which is examined in this study for the first time by a first-principles method. For this, crystal models for DFT calculations were designed by substituting Sr2+ ions for Ba2+ ions at 1b or 2d sites, assuming three main cases: substitution of Sr2+ for only Ba2+ ions at 1b sites (case 1), Sr2+ substitution for only Ba2+ ions at 2d sites (case 2), and Sr2+ distribution at both 1b and 2d sites by fifty–fifty (case 3). Various configurations of crystal models with different ionic distributions were considered for each case and the energy was calculated.
m1 space group, in which Na+ ions occupy 1a sites and Ba2+ ions 1b sites, and 2d sites are occupied by 50% of Na+ and 50% of Ba2+ ions.38 However, the distribution of ions in NSBP has not yet been identified, which is examined in this study for the first time by a first-principles method. For this, crystal models for DFT calculations were designed by substituting Sr2+ ions for Ba2+ ions at 1b or 2d sites, assuming three main cases: substitution of Sr2+ for only Ba2+ ions at 1b sites (case 1), Sr2+ substitution for only Ba2+ ions at 2d sites (case 2), and Sr2+ distribution at both 1b and 2d sites by fifty–fifty (case 3). Various configurations of crystal models with different ionic distributions were considered for each case and the energy was calculated.
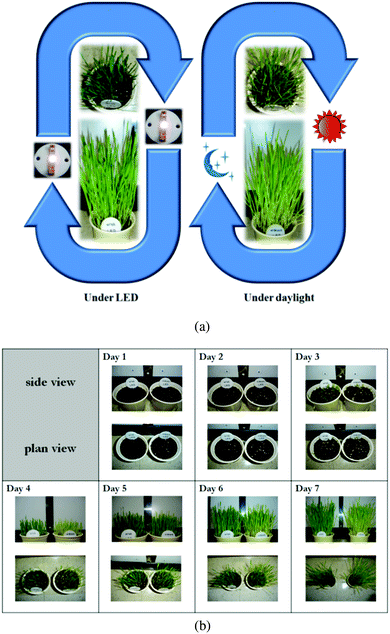
The DFT calculation results revealed that the energy of the crystal models belonging to case 2 was overall stable with a lower energy than those of the other cases. In this case, Sr2+ ions reside only at 2d sites so the 2d site is occupied by Sr2+ and Na+, and the 1b site is filled only with Ba2+ ions. The energy of the crystal models of case 1 was the highest among the three cases. With the assumption that the chance of the appearance of each crystal model is proportional to the Boltzmann factor, and that the overall crystal structure of NSBP can be composed from the ideal mixing of the crystal models according to their probability, equations for the evaluation of Sr2+ distribution in NSBP are established as follows:39
 | (1) |
 | (2) |
where RSr1b and RSr2d are the proportion of Sr2+ ions residing in 1d and 2d sites, respectively, and P denotes the partition function that is the sum of all the Boltzmann factors of the crystal models. The energy term that must be input in the Boltzmann factor was obtained by subtracting the energy of the most stable crystal model (i.e., the minimum energy) from each crystal model's energy (Ei,j,k − Emin of the equations). ×0.5 was applied for case 3 in the equations because Sr2+ occupied 50% of the 1b or 2d sites.
The sigma terms in eqn (1) and (2), that is, the probability of cases 1, 2 and 3 were calculated to be 0.3%, 87.8%, and 11.9%, respectively; thus, RSr1b and RSr2d were obtained as 6.3% and 93.7%. The distribution of ions in NSBP obtained from the calculation is presented in Table 1 and the corresponding crystal structure is shown in Fig. S2 (ESI†). For the investigation of the doping sites for Eu2+ and Mn2+ in the NSBP crystal, the energies of the crystal models were calculated for changes in the location of the dopants. The results were used for the estimation of Gibbs free energy change (ΔG) for the corresponding doping reaction assuming the conditions of 1450 K in a hydrogen atmosphere (Table 2). The results indicated that Eu2+ could substitute both Ba2+ in the 1b site and Sr2+ in the 2d site, being expected to have a higher doping probability at the 2d site due to a lower ΔG in the doping reaction in this site. While the ΔG of Mn2+ doping was higher than in the Eu2+ cases, we expected that the doping limit of Mn2+ would be much lower than that of Eu2+. The ΔG for the substitution of Sr at 2d was the lowest also for Mn2+. Substitution of the ions for Na with or without the formation of VNa was also considered, but the ΔG was higher than doping at the 2d site. Thus, Eu2+ and Mn2+ in the Na(Sr0.5Ba0.5)PO4 phosphor were preferably doped in the 2d site, as shown in Fig. 2.
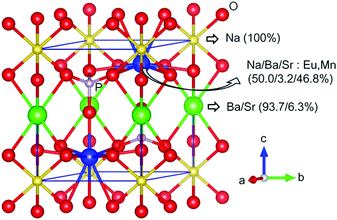 | ||
| Fig. 2 Crystal structure of the Eu2+ and Mn2+ codoped Na(Sr0.5Ba0.5)PO4 based on the DFT calculation results. | ||
The specific wavebands as well as the daily dose of light have a profound effect on plant growth. In general, red and blue light best drive photosynthetic metabolism associated with autotrophic growth habits.25 Photosynthetically inefficient light qualities also impart important environmental information to a developing plant. Additionally, the successful production of many plants requires an understanding of how they respond to the photoperiod, how the photoperiod changes during the day/year, and how to modify the photoperiod to control growth and development. In our scheme, by simply adjusting the doping content of Eu2+ and Mn2+ in customized single host LEDs, as shown in Fig. S1 (ESI†), we aim to produce more energy-efficient lighting that directly impacts plant growth.
The luminescence degradation of a phosphor is usually caused by thermal, chemical or hydrolytic attacks. Degradation beyond a certain degree required by practical applications significantly reduces the reliability and shortens the lifetime of wLEDs even though they have high quantum efficiency and useful emission colors.
The temperature dependence of phosphors used in phosphor-converted LEDs is important in understanding the influence on the light output and energy transfer in the co-activator as well. Energy transfers from the sensitizer to the activator through phonon-assisted tunneling revert to the ground state to give a shorter wavelength emission.20 Therefore, the energy transfer efficiency between the sensitizer and the activator is strongly dependent on the energy barrier ΔE, and the temperature T is a key parameter governing both the CIE value and the emission band spectrum of the corresponding single component white-emitting phosphors.
Here, we investigate the thermal stability of the given phosphors, Eu2+/Mn2+ codoped NSBP and CMP, by recording the photoluminescence spectrum of the samples heated up to 200 °C while held at each temperature for several minutes. These measurements help us to understand both the moisture/humidity resistance and the thermal degradation mechanism of the phosphor being investigated. Generally, Eu2+ can transfer its absorbed energy fully or partially to Mn2+ in many Eu2+ and Mn2+ codoped hosts. The temperature variable emission spectra for the given phosphors are presented in Fig. 3a and b. The emission intensity gradually declined with the temperature; this decline is presented in the configurationally coordinate diagram. Interestingly, our results clearly show that the degree of thermal quenching of the two phosphors was slightly different, which might be related to the energy transfer efficiency as well as to the resulting color purity, as shown in Fig. 3c.
In CMP:Eu2+,Mn2+, the blue emission band originating from the Ca2+-site substituted Eu2+ activator exhibits a much faster thermal quenching behavior, and hence produces a red-shifting CIE value. This result can be understood by the crystallographic property of the CMP host compound.
The crystal structure of CMP is monoclinic and its space group is C2/c. Within the lattice, there are five different Ca2+ sites that can be substituted by Eu2+ as an activator. Each Ca2+ site has different structural information, such as coordination numbers, which mainly affects the environment of the activators. Recently, we found a similar quenching behavior of Eu2+/Mn2+ activated phosphors: alkali earth metal phosphates from the SrO–MgO–P2O5 ternary system. Both the band position and half width of the Mn2+-induced emission spectrum are mainly concerned dissimilar neighboring states around the Mn2+ ion within the intermixed alkali earth metal (e.g., Sr2+/Mg2+) sites in various hosts, which result in widely tunable colors from violet-red through orange-red to pure red.37 Thus, the CMP-based phosphor has site-sensitive characteristics on which the luminescence properties and thermal stability are dependent on each other, because Eu2+ and a variable amount of Mg2+ tend to substitute for the same Ca2+ sites.
As seen in Fig. 3d, the thermal quenching data were fitted to a modified Arrhenius eqn (3),17,40
 | (3) |
To demonstrate the LED chip-in-package performance of the given Eu2+ and Mn2+ coactivated orthophosphates, we fabricated an LED lamp using a nUV-chip overcoated with the given phosphor samples. The specific phosphor overcoated LED fabrication procedure and the emission spectra of a nUV-LED (λem ∼ 395 nm) are described in our previous papers.36,37
The phosphor samples were coated onto UV LED chips with a maximum excitation wavelength of 380–385 nm. Inset figures show the captured image of the illuminating LED with the corresponding phosphors under an applied power of ∼300 mW. Fig. 4a–d show the electroluminescence (EL) spectra of the given NSBP- and CMP-phosphor overcoated wLEDs. Both blue/red emission bands are clearly located in the region of 450–475 and 580–625 nm.
Clearly, we see an additional short wavelength emission band positioned at 385 nm of the NSBP:Eu2+,Mn2+ phosphor converted LEDs induced by the transmitted nUV excitation source. The EL intensity gradually increased with the applied forward bias current. This indicates that neither of the orthophosphate-based white LEDs experiences spectral saturation, and thus we regard the mixed phosphors as promising candidates for UV LED-based white LED applications. In addition, there were slight CIE value changes with an increasing applied current of (±0.017, ±0.013) at NSBP and (±0.010, ±0.015) at CMP, indicating that the resultant wLEDs had a good color stability, properly tuned to the maximum photosynthetic reactions.
Fig. 4e and f shows the stability of the electroluminescence intensity of the developed phosphate converted-LEDs with an illuminating lamp image. It is clear that the emission intensity gradually increases and becomes stable over time for both NSBP and CMP phosphors. More specifically, the relative emission intensity of the corresponding NSBP coated pc-LEDs is much higher than that of other alkali earth metal based orthophosphates, NaMePO4 (Me = Mg, Ca, Sr, Ba).42 Furthermore, a stable luminescence under chip-in-package conditions represents an excellent thermal quenching property even with phosphors other than the commercial YAG:Ce3+ phosphor, as shown in Fig. 3d. Consequently, excellent thermal quenching properties of Eu2+ and Mn2+ codoped NSBP and CMP phosphors were observed, which supports their promising application as cost effective pc-wLEDs for ongoing solid state lighting.
Finally, we tested the influence of spectral quality on plant growth using the given orthophosphate-overcoated LED lighting. Note that the Na(Sr0.5Ba0.5)PO4 phosphor with a 0.05 Eu2+ and 0.05 Mn2+ mole concentration was used for the pc-LEDs, which means the tailored red/blue emission band position and relative intensity produced light sources that stimulated plant growth well.
The quality, intensity, and duration of light directly impact plant growth. The light quality refers to the color or wavelength that reaches the plant's surface and is a major consideration for indoor growing. Red and blue rays have the greatest impact on plant growth, while green light is least effective (the reflection of green light gives the green color to plants); blue light is primarily responsible for vegetative leaf growth and red light, when combined with blue light, encourages flowering.
The fluorescent cool white lamps are high in the blue range and are typically the best choice for starting seeds indoors. For flowering plants that need more red light, broad spectral fluorescent bulbs are most effective. Incandescent lights are high in red and red-orange, but generally produce too much heat for use in supplementing plant growth.41–44
Under conditions of naturally short days, long days can be created by lighting at the end of the day, which is known as day-extension, or by lighting during the middle of the night, which is known as night-interruption. For day-extension lighting, lamps should be turned on around sunset and remain on until the desired photoperiod is completed.
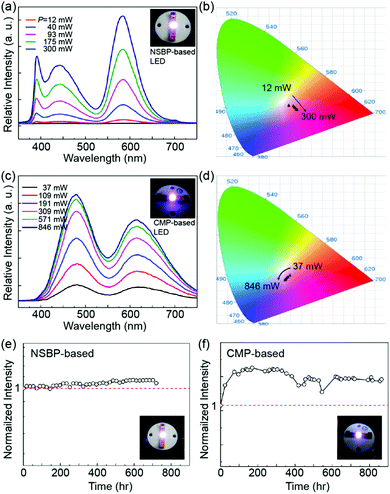
First, as shown in Fig. S3 (ESI†), NSBP-overcoated LEDs were evaluated preliminarily for stimulating the growth of onions. We performed an experiment with natural daylight conditions (12 h or less) and then with a phosphor-converted LED that illuminated the plants continuously all night. Note that the reference plant was grown only on the daylight on/off cycle; i.e., it was solely dependent on sunlight.
The phosphor-converted LED activated plant experienced a faster germination and grew more quickly and healthily. These results clearly indicate that proper wavelength-tailored artificial lighting efficiently delivers the spectral outputs, and therefore the day length can be extended with artificial lighting, which maintains the photosynthesis reaction of indoor plants.
We also investigated the preliminary lighting effect on indoor plant growth using other hydroponics mode plants, such as oats. Oats can be easily produced hydroponically in a week, from seed germination to harvest. Since one of the critical factors for growing oats is temperature, we maintained the temperature within the unit at 25–30 °C as well as the humidity, regulated by a custom-made controller. The set up image of the thermo-hygrostat chamber we used is appended in the ESI† as Fig. S4. To fully evaluate the given LEDs as artificial lighting, four LEDs with spacings of several centimeters were mounted, and the oats were illuminated continuously all day long. The reference plant was grown under only the daylight-on/off cycle as in the onion test. Similar to onions, we can see in Fig. 5 (presenting both comparative plane/side view images and daily panoramic images) that the Eu2+/Mn2+ activated artificial lighting can be very effective in enhancing the overall photosynthesis reaction, i.e., the oats grown under 24 h-illuminating LEDs have abundant greenery with fast germination. On the other hand, the oats grown under activated daylight-only conditions withered fast with a low growth and development.
To support the superiority of the given NSBP-LEDs, we carried out supporting experiments in the same conditions using the Eu2+-activated red-emitting phosphors to determine how the spectral distribution affects the photobiological interactions. Overall luminescent properties of the tested phosphor, Ba2Mg(BO3)2:Eu2+, are reported elsewhere36 (please see Fig. S5, ESI† for reference). As shown in Fig. 6, we can realize that only red-illuminating light sources are less effective in cultivating oats; sprouts shot up rather sluggishly with whitening in their bases marked as dotted circle, which made them look withered. Thus, the optimal light output for blue light acted as a signal triggering the response with the complementing red right, which is essential to promoting the overall photosynthetic reactions. The results reveal that oats grown under 24 h-illuminating Eu2+/Mn2+ activated artificial lighting LEDs have abundant greenery with a fast germination. These results clearly show that controlled-blue/red emission readily realized using single compound pc-LED lighting can effectively accelerate plant growth and enhance the yield and quality of agricultural products.
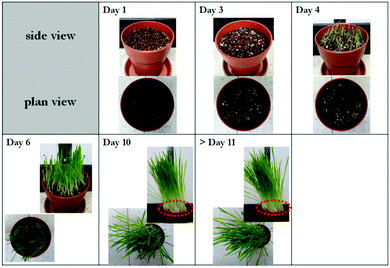 | ||
| Fig. 6 Daily-tracked panoramic images of the oats grown under illuminating Ba2Mg(BO3)2:Eu2+ converted-LEDs. The excitation and emission spectra, and CIE values of LED using the tested phosphor, Ba2Mg(BO3)2:Eu2+, are reported in our previous work, ref. 38. | ||
Conclusion
In summary, we successfully designed cost/energy-efficient smart LED lighting using highly luminescent color tunable phosphates for urban indoor plant factories. The resultant unique supplemental lighting can enhance the overall photosynthesis reaction for indoor plants. The lighting offers very effective “blue (λmax = 450 nm)/red (λmax = 620 nm)” two band emissions, which are encouraging results to the most electrically efficient colors via single composition phosphor-activated LEDs.To develop promising lighting for indoor plants, we investigated the luminescence (especially thermal quenching) of Eu2+ and Mn2+ codoped blue/red illuminating orthophosphates. Dual-band emission, which can be easily tuned by changing the host composition and activator concentration, properly works both for vegetative growth (∼450 nm) and for growing fruits or flowers (∼600 nm). The temperature/power variable emission spectral behavior of both the sensitizer and the activator ions was found to be mainly dependent on the host compound, and thus we can meet the purposes of illuminating elements by designing the appropriate chemical composition of a Mn2+-activated orthophosphate phosphor with the optimum concentration of the sensitizer ion, Eu2+. We have demonstrated the unique dual-band emission of the given phosphates with an excited UV LED chip-in-package test that revealed an enhancing overall photosynthesis reaction, i.e., the oats grown under 24 h-illuminating LEDs had abundant greenery with a fast germination.
These results suggest that proper wavelength-tailored artificial lighting is effective in efficiently delivering spectral outputs. Also, day-length lighting can be extended with artificial lighting that maintains the photosynthesis reaction of indoor plants. This strategy may evolve into an even more effective system when more controlled growth conditions, such as humidity, nutrient agents and precisely customized composition of light, are taken into consideration.
Author contributions
The manuscript was written through contributions of all the authors.Competing financial interest
The authors declare no competing financial interest.Acknowledgements
The present work is a joint research project. The authors appreciate the financial support from the ISTK (Korea Research Council for Industrial Science and Technology).Notes and references
- K. Inada, Plant Cell Physiol., 1976, 17, 335–365 Search PubMed
.
- K. J. McCree, Agric. Meteorol., 1972, 9, 191–216 CrossRef
.
- K. M. Folta and S. A. Maruhnich, J. Exp. Bot., 2007, 58, 3099–3111 CrossRef CAS PubMed
.
- I. Terashima, T. Fujita, T. Inoue, W. S. Chow and R. Oguchi, Plant Cell Physiol., 2009, 4, 684–697 CrossRef PubMed
.
- K. R. Cope, M. C. Snowden and B. Bugbee, Photochem. Photobiol., 2014, 90, 574–584 CrossRef CAS PubMed
.
- S. Ye, F. Xiao, Y. X. Pan, Y. Y. Ma and Q. Y. Zhang, Mater. Sci. Eng., R, 2010, 71, 1–34 CrossRef
.
- L. Chen, C. C. Lin, C. W. Yeh and R. S. Liu, Materials, 2010, 3, 2172–2195 CrossRef CAS
.
- R. C. Morrow, HortScience, 2008, 43, 1947–1950 Search PubMed
.
- G. D. Massa, H. Kim, R. M. Wheeler and C. A. Mitchell, HortScience, 2008, 43, 1951–1956 Search PubMed
.
- J. K. Han, J. I. Choi, A. Piquette, M. Hannah, M. Anc, M. Galvez, J. B. Talbot and J. McKittrick, ECS J. Solid State Sci. Technol., 2013, 2, R3138–R3147 CrossRef CAS
.
- M. D. Lago, M. Meneghini, N. Trivellin, G. Mura, M. Vanzi, G. Meneghesso and E. Zanoni, Microelectron. Reliab., 2012, 52, 2164–2167 CrossRef
.
- M. Raukas, J. Kelso, Y. Zheng, K. Bergenek, D. Eisert, A. Linkov and F. Jermann, ECS J. Solid State Sci. Technol., 2013, 2, R3168–R3176 CrossRef CAS
.
- J. McKittrick, M. E. Hannah, A. Piquette, J. K. Han, J. I. Choi, M. Anc, M. Galvez, H. Lugauer, J. B. Talbot and K. C. Mishra, ECS J. Solid State Sci. Technol., 2013, 2, R3119–R3131 CrossRef CAS
.
- Y. Huang, Y. Cao, C. Jiang, L. Shi, Y. Tao and H. J. Seo, Jpn. J. Appl. Phys., 2008, 47, 6364–6368 CrossRef CAS
.
- Y. S. Tang, S. F. Hu, C. C. Lin, N. C. Bagkar and R. S. Liu, Appl. Phys. Lett., 2007, 90, 151108 CrossRef
.
- B. K. Grandhe, V. R. Bandi, K. Jang, S. Kim, D. Shin, Y. Lee, J. Lim and T. Song, J. Alloys Compd., 2011, 509, 7937–7942 CrossRef CAS
.
- S. Zhang, Y. Nakai, T. Tsuboi, Y. Huang and H. J. Seo, Inorg. Chem., 2011, 50, 2897–2904 CrossRef CAS PubMed
.
- C. C. Lin, Z. R. Xiao, G. Y. Guo, T. S. Chan and R. S. Liu, J. Am. Chem. Soc., 2010, 132, 3020–3028 CrossRef CAS PubMed
.
- S. Zhang, D. Wei, R. Zhu, Y. Huang and H. J. Seo, Ceram. Int., 2011, 37, 3697–3702 CrossRef CAS
.
- K. Li, M. Shang, H. Lian and J. Lin, J. Mater. Chem. C, 2016, 4, 5507–5530 RSC
.
- C. H. Huang, T. M. Chen, W. R. Liu, Y. C. Chiu, Y. T. Yeh and S. M. Jang, ACS Appl. Mater. Interfaces, 2010, 2, 259–264 CAS
.
- W. Wu and Z. Xia, RSC Adv., 2013, 3, 6051–6057 RSC
.
- D. Geng, M. Shang, Y. Zhang, H. Lian and J. Lin, Dalton Trans., 2013, 42, 15372–15380 RSC
.
- L. Ma, D. Wang, Z. Mao, Q. Lu and Z. Yuan, Appl. Phys. Lett., 2008, 93, 144101 CrossRef
.
- T. Nakajima and T. Tsuchiya, ACS Appl. Mater. Interfaces, 2015, 7, 21398–21407 CAS
.
- J. Chen, C. Guo, Z. Yang, T. Li and J. Zhao, J. Am. Ceram. Soc., 2016, 99, 218–225 CrossRef CAS
.
- Z. Mao, J. Chen, J. Li and D. Wang, Chem. Eng. J., 2016, 284, 1003–1007 CrossRef CAS
.
- G. Kreese and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef
.
- J. P. Perdew, K. Bruke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed
.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. D. Corso, S. Fabris, G. Fratesi, S. Gironcoli, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed
.
- Y. Kim, J. Kim and S. Kang, J. Mater. Chem. C, 2013, 1, 69–78 RSC
.
- L. Wang, T. Maxisch and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 195107 CrossRef
.
- P. Dorenbos, ECS J. Solid State Sci. Technol., 2013, 2, R3001–R3011 CrossRef CAS
.
- R. A. McCauley, F. A. Hummel and M. V. Hoffman, J. Electrochem. Soc., 1971, 118, 755–759 CrossRef CAS
.
- J. Lu, F. Du, R. Zhu, Y. Huang and H. J. Seo, J. Mater. Chem., 2011, 21, 16398–16405 RSC
.
- S. J. Kim, Y. J. Yun, H. K. Jung and S. H. Choi, Opt. Lett., 2014, 39, 251–254 CrossRef CAS PubMed
.
- Y. J. Yun, H. J. Lim, J. S. Park, M. Wu, H. K. Jung and S. H. Choi, Dalton Trans., 2015, 44, 338–344 RSC
.
- C. Calvo and R. Faggiani, Can. J. Chem., 1975, 53, 1849 CrossRef CAS
.
-
D. A. McQuarrie, Statistical Mechanics, University Science Books, 2000, ch. 4 Search PubMed
.
- I. Baginskiy and R. S. Liu, J. Electrochem. Soc., 2009, 156, G29–G32 CrossRef CAS
.
- C. S. Brown, A. C. Schuerger and J. C. Sager, J. Am. Soc. Hortic. Sci., 1995, 120, 808–813 CAS
.
- R. J. Bula, R. C. Morrow, T. W. Tibbitts, D. J. Barta, R. W. Ignatius and T. S. Martin, HortScience, 1991, 26, 203–205 CAS
.
- K. Fujiwara and T. Sawada, J. Light Visual Environ., 2006, 30, 170–176 CrossRef
.
- D. J. Tennessen, E. L. Singsaas and T. D. Sharkey, Photosynth. Res., 1994, 39, 85–92 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Crystal structure, photoluminescence spectra, and experimental apparatus are included. See DOI: 10.1039/c7cp00742f |
| This journal is © the Owner Societies 2017 |