Controlled ring-opening polymerisation of cyclic phosphates, phosphonates and phosphoramidates catalysed by heteroleptic BHT-alkoxy magnesium complexes†
Abstract
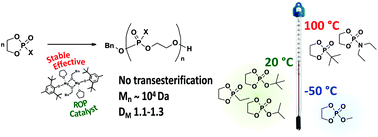
We report here that the heteroleptic BHT-Mg(OR) complex, i.e. [(BHT)Mg(OBn)(THF)]2 (Mg3), represents an effective and versatile ring-opening polymerisation (ROP) catalyst for several cyclic ethylene phosphate monomers (CEPMs), such as ethylene phosphates with methoxy- (MeOEP, 1), isopropoxy- (iPrOEP, 2), and tert-butoxy- (tBuOEP, 3) substituents, N,N-diethyl ethylene phosphoramidate (Me2NEP, 4) as well as ethyl (EtPPn, 5) and tert-butyl (tBuPPn, 6) ethylene phosphonates. Mg3 retains its catalytic activity over a broad temperature range from −50 to 100 °C and efficiently carries out the fast and controlled polymerisation of CEPMs with sterically unhindered alkoxy groups with less than 1% chain branching even at near-complete conversion. Compared with ROP catalysed by 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), Mg3 performs much better in the polymerisation of CEPMs with bulky groups and of monomers sensitive to strong nucleophiles, such as tBuOEP.


 Please wait while we load your content...
Please wait while we load your content...