Suppression of contact hypersensitivity after repeated exposures of humans to low doses of solar simulated radiation
Dr.
Joanna
Narbutt
*a,
Aleksandra
Lesiak
a,
Malgorzata
Skibinska
b,
Anna
Wozniacka
a,
Henk
van Loveren
c,
Anna
Sysa-Jedrzejowska
a,
Iwona
Lewy-Trenda
d,
Aleksandra
Omulecka
d and
Mary
Norval
e
aDepartment of Dermatology, Medical University of Lodz, Krzemieniecka 5, Lodz, Poland. E-mail: joanna.narbutt@onet.pl; Fax: +4842 688 4565; Tel: +4842 688 4565
bDepartment of Dermatology, Basildon General Hospital, Essex, England, UK
cNational Institute of Public Health and Environment, Bilthoven, The Netherlands
dDepartment of Pathomorphology, Medical University of Lodz, Lodz, Poland
eMedical Microbiology, University of Edinburgh Medical School, Scotland, UK
First published on 15th June 2005
Abstract
Although it is generally recognised that UV radiation (UVR) can induce suppression of contact hypersensitivity (CHS) in human subjects, most protocols to date have not tested the effect of low daily doses of solar simulated radiation (SSR). In the present study, healthy individuals, divided into four groups each consisting of approximately 34 subjects, were whole-body irradiated with 1.2 standard erythema doses of SSR for 2, 10 or 30 consecutive days, or were unirradiated. They were sensitised with diphenylocyclopropenone (DPCP) on one exposed body site 24 h after the final UVR. The occurrence and severity of the primary allergic response were noted, and both parameters were shown to be significantly lowered in the group irradiated for 30 days compared with the unirradiated group. Elicitation of CHS was undertaken 3 weeks after the sensitisation, using a range of concentrations of DPCP on a UV-protected body site. The extent of the CHS at 48 h was assessed by the clinical score, by an erythema meter and by histological examination of a biopsy taken from the site challenged with one selected concentration of DPCP. Although erythema and pigmentation did not differ between the groups, a significant negative correlation was found between the clinical CHS score and the number of days of UV exposure, at the lowest challenge dose of DPCP. In addition a significant negative correlation was revealed between the intensity of spongiosis (intraepidermal oedema and vesicles, as evaluated by histology) and the number of days of UV exposure. Thus small daily doses of SSR induce suppression of CHS in human subjects and the effect is cumulative, indicating that there is no adaptation to the immunomodulating effects of UVR, at least over the test period of 30 days.
Introduction
It is recognised that exposure to UV radiation (UVR) causes sunburn, ocular damage and photoageing and is a major risk factor for the induction of skin cancers; it can also modulate immune responses in both animal models and in humans. 1–4 On the other hand, UVR is required for the synthesis of vitamin D, being in the sun often promotes a sense of well-being, and phototherapy is used successfully in the treatment of various dermatoses, such as psoriasis and atopic dermatitis.UV-induced immunosuppression occurs by a complex process including changes in the production of cytokines by keratinocytes, such as IL-10, IL-6 and TNF-α, in the expression of adhesion molecules such as ICAM-1 and in the morphology and function of Langerhans cells (LCs) and dendritic cells (DCs).5,6 Modulation of the contact hypersensitivity (CHS) reaction is frequently used to evaluate the immune response following UV exposure. In the sensitisation phase, a hapten, such as diphenylocyclopropenone (DPCP) is applied to the skin and is taken up by LCs locally. The LCs are stimulated to migrate to the draining lymph nodes where they present the sensitiser to appropriate T lymphocytes, thus initiating the specific immune response. In the elicitation phase, the sensitiser is reapplied epi-cutaneously resulting in the generation of a delayed T cell response, manifest by a powerful inflammatory reaction in the local site. In human beings, the sensitiser is often applied to an area of the buttock skin, and a primary allergic response (PAR) frequently develops 7–14 days later, in subjects who have not previously come in contact with the particular sensitiser. Some weeks later the individual is challenged by a second exposure to a range of concentrations of the sensitiser, applied to another skin site, often on the inner arm. The CHS is maximal after 48 hours. The intensity of the elicitation phase varies from a mild erythema to a severe spreading response with blistering. It can be measured by several methods but in clinical practice this is usually done by visual scoring.7
Most of the original experiments evaluating the suppression of CHS by UVR used sources emitting a higher proportion of UVB to UVA (often more than 50% UVB) compared with that found in sunlight, which contains about 3.4% UVB and 96.6% UVA.8 Some more recent studies have used solar simulated radiation (SSR) and have shown that a single exposure to a dose of SSR representing more than one minimal erythema dose (MED) suppresses CHS.9–13 In another instance it was demonstrated that a single dose of SSR, lower than 1 MED (0.25–0.5 MED) could also suppress the CHS response, although this was only apparent in individuals with skin type I/II and not in those with skin type III/IV.14 To our knowledge no study thus far has evaluated the effect of repeated daily exposures to low doses of SSR on the induction of immune responses in human subjects, as assessed by CHS. This pattern of irradiation hopefully mimics natural conditions more closely than a single large exposure as the majority of people are out in the sun for short periods of time on a daily basis over the summer months.
It was our main aim to assess CHS in groups of people whole-body irradiated with a low dose, 1.2 standard erythema dose (SED),15 of SSR on each of 2 days, 10 days or 30 days. The Cleo Natural lamps, which emit a UV spectrum closely resembling that emitted by the sun, were used. In order to obtain the most reliable results, three methods were undertaken to measure the extent of CHS, namely visual scoring, a reflectance device measuring erythema and pigmentation, and the morphology of skin biopsies.
Materials and methods
Subjects and UV irradiation
The study group consisted of 165 healthy volunteers with either II or III skin phototype, as assessed by the Fitzpatrick scoring system.16 We selected these phototypes as they are found in the majority of the population in Central Europe. The subjects were recruited following advertising in the local newspapers and within the University. They were without any skin or other disease and were not receiving any medication. Subjects exposed to high doses of sunlight or sunlamps within two months prior to the study or previously exposed to the contact allergen, DPCP, were excluded. To decrease the influence of natural solar radiation, all the procedures were performed between November and February of the following year. Each volunteer gave written informed consent before entry into the study, and the experimental plan was approved by the local Ethics Committee.The subjects underwent a thorough physical examination and blood count. Forty non-irradiated individuals (17 women, 23 men; mean age 25.0) acted as the control group (group 1). A hundred individuals were irradiated (whole body irradiation in two half-wall cabinets excluding the genital area) in three groups, group 2 consisting of 33 people (17 women, 16 men; mean age 26.1 years) irradiated for 2 consecutive days, group 3 consisting of 34 (13 women, 21 men; mean age 24.3 years) irradiated for 10 consecutive days, and group 4 consisting of 33 (16 women, 17 men; mean age 26.7 years) irradiated for 30 consecutive days with a dose of 1.2 SED (1 SED is equivalent to an erythemal radiant exposure of 100 J m−2) on each occasion. The SSR was generated by 100 W Cleo Natural lamps (Philips, Eindhoven, The Netherlands) giving an even field of irradiance (4% UVB, 96% UVA) of about 4.95 mW cm−2 on the skin surface at 20 cm from the source. The emission spectrum of the Cleo Natural lamp is shown in Fig. 1. Measurement of the intensity of the lamps was performed using a Solar Light 3D UV meter (Solar Light Co., Philadelphia, USA).
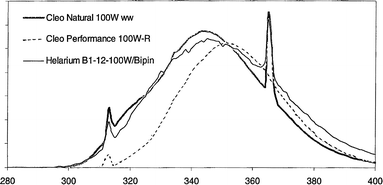 | ||
| Fig. 1 Emission spectrum for the Cleo Natural 100 W lamp. | ||
For histological analysis, another 25 individuals (12 women, 13 men, mean age 25.4) who were neither irradiated nor sensitised acted as negative controls for the histology study. Their skin samples were taken from the same body site as in the other groups.
Sensitisation and elicitation of CHS
Diphenylcyclopropenone (DPCP: Fluka Chemie GmbH, Buchs, Switzerland) was dissolved in acetone just before use. The subjects were sensitised on the left irradiated (groups 2–4) or non-irradiated (group 1) buttock skin using two 7 mm petrolatum-backed filter discs, one soaked in 20 µl 0.1% DPCP (20 µg) and the other in 20 µl acetone. The irradiated subjects were sensitised 24 h after the final exposure to SSR. The filters were mounted inside 8 mm aluminium Finn chambers (Epitest Ltd Oy, Tuusula, Finland) which were taped to the skin with hypo-allergenic Scanpore tape and left in place for 48 h. The volunteers were requested to keep the patch dry for 48 h, after which the patch was removed. The sensitisation site was assessed for the development of a PAR. Three weeks later, all the volunteers in groups 1–4 received an antigenic challenge on the unirradiated upper inner left arm skin using a series of 20 µl acetone containing 0.4, 0.8, 1.6, 3.2 and 6.4 µg DPCP placed on the discs. One additional patch contained acetone only as a control. The highest amount of DPCP was applied only if no PAR was observed. The patches were put on the skin in Finn chambers, held in place with tape, and were removed 6 h later. The sites were marked on each arm with surgical marker. The CHS response was evaluated after 48 h by using a reflectance device (see below), by histology (see below) and by a subjective visual scoring system: 0, no reaction; 1, macular erythema; 2, erythema with infiltration; 3, erythema with infiltration and papules or vesicles; and 4, bullous reaction. The visual score for all the subjects was determined by one dermatologist who did not know to which group the individual subject belonged at the time of the examination.Erythema and pigmentation
Erythema and pigmentation were quantified using the UV Optimise 555 device (ChromoLight, APS, Denmark).17,18 This patented device is used in dermatological practice to optimize UV treatements. The probe consists of two light emitting diodes, a photodetector and the circuitry necessary to collect and transmit data regarding the skin's reflection of red and green light to a microprocessor. The results obtained are a percentage of the reflection of 558 and 660 nm light. Erythema and pigmentation are determined from these readings and automatically calculated independently of each other. Each site of elicitation plus the control site was measured three times (groups 1–4). In addition measurements were taken before and after irradiation, but before sensitisation, on the buttock skin in the UV-exposed groups (groups 2–4). The mean values of the three readings were calculated.Histological examination
A 3 mm-punch skin biopsy was taken from the 3.2 µg DPCP site in each subject 48 h after elicitation. Additionally, 3 mm punch skin biopsies were taken from the same skin site of the 25 non-sensitised, non-irradiated volunteers. The tissue specimens were fixed in 10% buffered formaldehyde, routinely processed for paraffin embedding and 3–4 µm sections cut. These were dewaxed and stained with haematoxylin and eosin. Ten serial sections were evaluated from each specimen by two independent pathologists at a magnification of 400x (Olympus Bxsystem microscope, Olympus Optical Co., LTD, Tokyo, Japan). Histological morphometry was performed using an image analysis system consisting of an IBM-compatible computer equipped with an optical mouse, Indeo Fast card (frame grabber, true-colour, real-time), produced by Indeco (Taiwan) and colour TV camera (Panasonic, Japan) linked to a Carl Zeiss Jeneval microscope (Germany). This system was programmed (programme MultiScan 8.08, Computer Scanning System, Poland) to calculate the intensity of spongiosis in the whole specimen and the minimum, maximum and mean thickness of the epidermis in the whole specimen.In each group of the volunteers, the histological analysis included an assessment of the following parameters: total thickness of the epidermis (mean value obtained from the 10 serial sections), the presence of the intraepidermal vesicles and intensity of spongiosis in the 10 serial sections graded as: 0, no spongiosis; 1, slight oedema, no intraepidermal vesicles; 2, oedema and single intraepidermal vesicles; and 3, severe oedema and multiple intraepidermal vesicles. The results obtained from the sensitized, irradiated individuals were compared with the sensitised, unirradiated individuals. The same parameters were examined in non-sensitised, non-irradiated volunteers.
Statistical analysis
The statistical analysis of the data was performed using the Mann–Whitney test, Wilcoxon pair test, χ2 test and non-parametric correlation of Spearman. A p value of less than 0.05 was considered as statistically significant.Results
Response of the subjects to SSR
The age, gender and phototypes of the subjects in groups 1–4 are shown in Table 1. No erythema was visible by eye in any individual 24 h after each exposure to the Cleo Natural lamps and also after the final irradiation. A light tan developed after the 30 UV exposures in the individuals in group 4. Statistical analysis of the erythema values measured with the UV Optimize 555 device revealed no significant differences before and after the final irradiation within each group or between the groups (p > 0.05). Analysis of the pigmentation values before and after the last irradiation gave significant differences within each of the groups (p = 0.004 for group 2, p = 0.003 for group 3 and p = 0.001 for group 4), but there were no differences between the groups (p > 0.05). These results are shown in Fig. 2.| No of group | No of subjects | Mean age years ± SD | Gender F/M | Phototype II/III |
|---|---|---|---|---|
| Unirradiated (group 1) | 40 | 25.0 ± 3.5 | 17/23 | 19/21 |
| SSR for 2 days (group 2) | 33 | 26.1 ± 7.3 | 17/16 | 17/16 |
| SSR for 10 days (group 3) | 34 | 24.3 ± 6.6 | 13/21 | 22/12 |
| SSR for 30 days (group 4) | 33 | 26.7 ± 7.4 | 16/17 | 22/11 |
 | ||
| Fig. 2 Skin pigmentation (mean group response) before and after exposure of groups of subjects to solar simulated radiation for 2, 10 or 30 consecutive days. | ||
Visual assessment of PAR and CHS
PARs were detectable on average 10 days after sensitisation. The response ranged from erythema with definite borders to strong erythema with oedema and blistering. The diameter varied between 11–25 mm. The percentage of subjects in groups 1–4 exhibiting PAR, those with blistering and the period of PAR following sensitisation are shown in Table 2. There were no statistically significant differences between groups 1–4 regarding the time point at which PAR first appeared and how long it lasted (p > 0.05). However the percentage of subjects in group 4 with PAR was significantly less than in group 1 (p = 0.03; χ2 test), and similarly the percentage of subjects in group 4 with a blistering response was significantly less than in group 1 (p = 0.03; χ2 test). The differences between groups 2 and 3 and group 1 for the same two parameters were not significant. Some of the subjects did not develop a PAR but they demonstrated CHS following elicitation. Thus the lack of PAR did not necessarily indicate a failure to sensitise. We observed clinically that the presence of severe PAR was indicative of a high intensity of CHS.| Group | Unirradiated (group 1; n = 40) | SSR 2 days (group 2; n = 33) | SSR 10 days (group 3; n = 34) | SSR 30 days (group 4; n = 33) |
|---|---|---|---|---|
| % with PAR | 85.0 | 78.8 p1–2 > 0.05 | 76.5 p1–3> 0.05 | 64.0 p1–4 = 0.03 |
| % with blistering | 12.5 | 9.1 p1–2 > 0.05 | 2.9 p1–3 > 0.05 | 0 p1–4 = 0.03 |
| Days of PAR (mean) | 9.5 | 8.5 | 7.5 | 11 |
The CHS was first assessed visually using the scoring system outlined in the Materials and Methods. The highest elicitation concentration of DPCP (6.4 µg) was not included in the analysis as it was not used in all of the subjects. A statistical analysis of the visual assessment of the CHS revealed no difference between any of the groups at any of the DPCP concentrations. The sum clinical score obtained from adding together the CHS response in each of the 4 groups at all 4 DPCP concentrations also showed no difference (p > 0.05). However, when the CHS score was simplified by treating it as a binomial trait where 0 represents no reaction and 1 represent a response, differences between the groups were found, as shown in Fig. 3. After using Spearman statistics, a significant negative correlation (R = −0.2; p = 0.015) was seen between the CHS score and the number of days of irradiation when 0.4 µg DPCP was used as the challenge dose (Fig. 3A). Although similar trends were found for the other three DPCP concentrations used (Fig. 3B–D), the differences between the groups were not statistically significant (results not shown).
 | ||
| Fig. 3 Frequency of CHS response, assessed by visual score (0, no response; 1, any response), to (A) 0.4, (B) 0.8, (C) 1.6 and (D) 3.2 (g DPCP in groups of subjects exposed to solar simulated radiation for 2, 10 or 30 consecutive days. | ||
CHS assessed by erythema and pigmentation
There were higher erythema values in response to the elicitation doses of 0.4, 0.8, 1.6 and 3.2 µg DPCP compared with the acetone site in groups 2 and 3 (p < 0.05) and in response to 0.8, 1.6 and 3.2 µg DPCP compared with the acetone site in group 4. However when the erythema values following elicitation were compared between groups 1–4, no statistically significant differences at any DPCP concentration were found. Similarly no significant differences between the groups regarding pigmentation were revealed (p > 0.05).CHS assessed by histology
The mean value for the thickness of the epidermis in the 25 healthy subjects who had not been UV-irradiated or sensitised was 0.061 mm. In groups 1–4, the mean thickness was 0.207, 0,166, 0.163 and 0.108 mm respectively following elicitation with a dose of DPCP of 3.2 µg. There was a statistically significant difference in epidermal thickness between group 1 and each of groups 2–4 (p < 0.05). The highest elicitating concentration of 6.4 µg DPCP was not analysed statistically as it was used only in subjects who did not developed a PAR.There was no spongiosis evident in the skin of the 25 unirradiated and unsensitised subjects. The highest intensity of spongiosis was observed in the group 1 subjects (unirradiated and sensitised). As shown in Fig. 4, the higher the number of days of SSR, the lower the intensity of spongiosis. When the number of days of SSR and the spongiosis score were treated as continuous variables, a negative correlation between these 2 factors was revealed (R = −0.28, p < 0.001, Spearman non-parametric test). In addition a significant correlation between the intensity of spongiosis and the clinical score for the CHS in response to 3.2 µg DPCP was found (p < 0.000001), and between the intensity of spongiosis and the sum clinical score for all the DPCP concentration was observed (p < 0.00004). When the spongiosis score was treated as a binomial trait, where 0 = no spongiosis and 1 = any reaction, and the χ2 statistical test (df = 3) was applied, differences were seen between the number of individuals with no reaction and the others. After using Spearman statistics, a significant negative correlation was found between the spongiosis intensity (0 or 1) and the number of days of SSR (R = −0.2, p = 0.01).
 | ||
| Fig. 4 The intensity of spongiosis in response to the challenge dose of 3.2 µg DPCP in skin samples from groups of subjects exposed to solar simulated radiation for 2, 10 or 30 consecutive days before sensitisation with DPCP: 0, no spongiosis; 1, slight oedema; no intraepidermal vesicles; 2, oedema and single intraepidermal vesicles; 3, severe oedema and multiple intraepidermal vesicles. | ||
Discussion
Response to repeated exposures to SSR
In our study, the groups of volunteers were whole-body irradiated with 1.2 SED SSR from the Cleo Natural lamps on a daily basis for up to 30 days. An exposure of 4 SED has been estimated to produce moderate erythema on unclimatised white skin or minimal erythema on previously exposed skin;8 thus 1.2 SED probably represents less than 0.3 MED for people with skin type II/III. As the ambient diurnal exposure on a clear sky summer day in Europe is 30–40 SED, a dose of 1.2 SED would be achieved in about 15 min of being out in the sun in the hours around midday. It is therefore a minimal exposure and was chosen to represent a natural situation for many individuals during the summer months. No evidence of erythema, oedema or blistering was found in any group following the SSR. Some pigmentation developed, although no significant difference in the extent of pigmentation between the groups irradiated for 2, 10 or 30 days was apparent.PAR
It is well known that a PAR can flare at the sensitisation site in some individuals 1–2 weeks after first contact with the hapten. This response is thought to indicate the onset of hapten-specific systemic sensitisation.19 It has been demonstrated previously that irradiating a localised skin site with a single dose of 3 MED SSR, followed by application of DPCP to that site, inhibited the development of PAR in 9/12 subjects, whereas 6/8 of the unirradiated controls developed PAR.8 Our data support this finding as we established that the greater the number of daily SSR exposures, the less likely the subjects were to exhibit PAR, and the less severe their PAR was (Table 2).It was found that some of the volunteers did not develop a PAR but still exhibited CHS following elicitation. Thus, as previously noted by Kelly et al.,9 the lack of a PAR did not indicate a failure to sensitise. Also, as reported by Kelly et al.,9 the severity of the PAR could be used to predict the intensity of the subsequent CHS response. For this reason the highest amount of DPCP (4.8 µg) was only used in the elicitation stage if no PAR was observed in the particular individual.
Assessment of CHS
Although the standard protocol in mice for monitoring the extent of the CHS is to measure oedema in the ear or footpad using a spring micrometer,20 such a uniform method has not been established as yet for human subjects. In the clinical setting, a visual score is frequently used to reflect the magnitude of the CHS response. However this system may be insufficiently objective or sensitive to demonstrate small changes, such as may occur following UVR. In the present study, to try to minimise possible discrepancies between the scores recorded by different observers, all the CHS responses were evaluated by a single experienced dermatologist who was unaware which group the subjects belonged to at the time of the examination.Another method involves a portable reflectance spectrometer which measures erythema, and the same instrument can also assess skin pigmentation by an independent melanin meter function. Such reflectance devices have been used successfully in measuring the CHS response to nickel21,22 and allow differences between individuals to be determined.23 However, in our study, we found that, when severe CHS responses occurred at sites of high DCPC concentration, the presence of oedema and blisters interfered with the readings and made the results unreliable. Such a conclusion was also reached by Kelly et al.9 Skin-fold thickness has been employed by others, such as Cooper et al.24 and Skov et al.,25 but such measurements are time-consuming and may vary with the operator, in addition to being problematic when oedema and blistering are present. Kelly et al.9 recommended the use of a high-frequency ultrasound scanner to determine dermal thickness at each elicitation site but we did not have access to such an instrument due to its high cost. We found that histological examination of biopsies taken from one of the series of elicitation sites gave reliable and sensitive results. Although this method is only semi-objective, examination of each skin section by two independent pathologists should reduce the possibility of error. Both epidermal thickness and the intensity of spongiosis were evaluated. Due to ethical considerations, only the 3.2 µg DPCP sites were biopsied.
Effect of repeated exposures to SSR on CHS
Our study included large groups of healthy individuals repeatedly exposed to small doses of SSR before sensitisation with DPCP and elicitation. From clinical scoring of the CHS response following elicitation with 0.4 µg DPCP and the histological appearance following elicitation with 3.2 µg DPCP, suppression of the CHS was induced by the SSR. This effect was dependent on the cumulative UV dose, as it became more apparent as the number of UV exposures increased. Based on our results, we suggest that there was no adaptation to the immunomodulation of the CHS response induced by low repeated doses of SSR. A similar conclusion was reached by Damian et al.21 who showed that the recall response to nickel in nickel-allergic volunteers was suppressed by exposure of a small area of the back to SSR and that this down-regulation persisted even after 4 weeks of repeated UV exposures. In contrast, if the irradiating source contained UVA only, the CHS returned to normal as the number of exposures increased.Previous studies in mice and human subjects have demonstrated that the suppression in the CHS induced by UVR is affected by many factors, for example, the UV dose, the area of skin irradiated, the timing of the sensitisation with respect to the UV exposure, and the hapten concentration and area of application.26–28 We chose to whole-body irradiate our volunteers with very small doses of SSR in an attempt to mimic a biologically relevant situation more accurately than irradiation of a limited area of skin only. It is recognised that the extent of CHS suppression is particularly dependent on the amount of hapten chosen for the sensitisation and the elicitation stages. We used DPCP at a sensitisation dose of 20 µg and elicitation doses ranging from 0.4–6.4 µg. It is possible that the effect of the SSR on the CHS response might have been larger if the elicitation doses had been lower. Kelly et al.9 recorded significant SSR-induced suppression with elicitation doses of 0.078, 0.156 and 0.31 µg DPCP following sensitisation with 15.6 µg DPCP.
In conclusion, daily exposure to a low dose of SSR over a period of 30 days can reduce the PAR and the CHS response to DPCP in human subjects, as assessed by clinical scoring and histology while no adaptation to these effects become evident. Further investigations are required to measure other types of innate and acquired immune responses under similar UVR conditions, particularly those thought to be important in the control of tumours and infectious diseases.
Acknowledgements
This study was funded by the European Union research project number QTL-CT-2001-00212IHA-UV.References
- J. Garseen and H. Van Loveren, Effects of ultraviolet exposure on the immune system, Crit. Rev. Immunol., 2001, 21, 359–397 Search PubMed.
- G. I. Harrison and A. R. Young, Ultraviolet radiation-induced erythema in human skin, Methods, 2002, 28, 14–19 Search PubMed.
- M. S. Duthie, I. Kimber I and M. Norval, The effects of ultraviolet radiation on the human immune system, Br. J. Dermatol., 1999, 140, 995–1009 CrossRef CAS.
- F. R. de Gruijl, J. Longstreth, M. Norval, A. P. Cullen, H. Slaper, M. L. Kripke, Y. Takizawa and J. van der Leun, Health effects from stratospheric ozone depletion and interactions with climate change, Photochem. Photobiol. Sci., 2003, 2, 16–28 RSC.
- S. Kodali, S. Beissert and R. D. Granstein, Physiology and pathology of skin photoimmunology, in Skin Immune System, ed. J. D. Bos, CRC Press, Boca Raton, FL, 3rd edn., 2004, pp. 457–474 Search PubMed.
- G. J. Clydesdale, G. W. Dandie and H. K. Muller, Ultraviolet light induced injury: immunological and inflammatory effects, Immunol. Cell Biol., 2001, 79, 547–568 CrossRef CAS.
- D. L. Damian and G. M. Halliday, Measurement of ultraviolet radiation-induced suppression of recall contact and delayed-type hypersensitivity in humans, Methods, 2002, 28, 34–45 Search PubMed.
- B. L. Diffey, Sources and measurement of ultraviolet radiation, Methods, 2002, 28, 4–13 Search PubMed.
- D. A. Kelly, S. L. Walker, J. M. McGregor and A. R. Young, A single exposure of solar simulated radiation suppresses contact hypersensitivity responses both locally and systemically in humans: quantitative studies with high frequency ultrasound, J. Photochem. Photobiol., B, 1998, 44, 130–142 CrossRef CAS.
- D. L. Damian, R. S. Barnetson and G. M. Halliday, Effects of low-dose ultraviolet radiation on in vivo human cutaneous recall responses, Australas. J. Dermatol., 2001, 42, 161–167 CrossRef CAS.
- D. A. Kelly, P. T. Seed, A. R. Young and S. L. Walker, A commercial sunscreen's protection against ultraviolet radiation-induced immunosuppression is more than 50% lower than protection against sunburn in humans, J. Invest. Dermatol., 2003, 120, 65–71 CrossRef CAS.
- E. D. Baron, A. Fourtanier, D. Compan, C. Medaisko, K. D. Cooper and S. R. Stevens, High ultraviolet A protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans, J. Invest. Dermatol., 2003, 121, 869–875 CrossRef CAS.
- P. Wolf, C. Hoffmann, F. Quehenberger, S. Grinschgl and H. Kerl, Immune protection factors of chemical sunscreens measured in the local contact hypersensitivity model in humans, J. Invest. Dermatol., 2003, 121, 1080–1087 CrossRef CAS.
- D. A. Kelly, A. R. Young, J. M. McGregor, P. T. Seed, C. S. Potten and S. L. Walker, Sensitivity to sunburn is associated with susceptibility to ultraviolet radiation-induced suppression of cutaneous cell-mediated immunity, J. Exp. Med., 2000, 191, 561–566 CrossRef CAS.
- B. L. Diffey, C. T. Jansen, F. Urbach and H. C. Wulf, The standard erythema dose: a new photobiological concept, Photodermatol. Photoimmunol. Photomed., 1997, 13, 64–66 CAS.
- T. B. Fitzpatrick, The validity and practicality of sun-reactive skin types I through VI, Arch. Dermatol., 1988, 124, 869–871 CAS.
- H. C. Wulf, A method and an apparatus for determining an individual's ability to stand exposure to ultraviolet radiation, patent PCT/DK, 1993, WO 93/16635:1-44 Search PubMed.
- A. B. Hansen, N. Bech-Thomsen and H. C. Wulf, Erythema after irradiation with ultraviolet B from Philips TL12 and TL01 tubes, Photodermatol. Photoimmunol. Photomed., 1994, 10, 22–25 CAS.
- C. Tseng, B. Hoffman, I. Kurimoto, T. Shimizu, G. J. Schmieder, J. R. Taylor and J. W. Streilein, Analysis of effects of ultraviolet B radiation on induction of primary allergic reactions, J. Invest. Dermatol., 1992, 98, 871–875 CrossRef CAS.
- V. E. Reeve, Ultraviolet radiation and the contact hypersensitivity reaction in mice, Methods, 2002, 28, 20–24 Search PubMed.
- D. L. Damian, R. Sc. Barnetson and G. M. Halliday, Low-dose UVA and UVB have different time courses for suppression of contact hypersensitivity to a recall antigen in humans, J. Invest. Dermatol., 1999, 112, 939–944 CrossRef CAS.
- A. A. Memon and P. S. Friedmann, Studies on the reproducibility of allergic contact dermatitis, Br. J. Dermatol., 1996, 134, 208–214 CrossRef CAS.
- J. Lock-Anderson, N. D. Knudstorp and H. C. Wulf, Facultative skin pigmentation in Caucasians: an objective biological indicator of lifetime exposure to ultraviolet radiation?, Br. J. Dermatol., 1998, 138, 826–832 CrossRef.
- K. D. Cooper, L. Oberhelman, T. A. Hamilton, O. Baadsgaard, M. Terhune, G. LeVee, T. Anderson and H. Koren, UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: relationship to dose, CD1a-DR+ epidermal macrophage induction and Langerhans cells depletion, Proc. Natl. Acad. Sci. USA, 1992, 89, 8497–8501 CAS.
- L. Skov, H. Hansen, J. N. W. N. Barker, J. C. Simon and O. Baadsgaard, Contrasting effects of ultraviolet-A and ultraviolet-B exposure on induction of contact hypersensitivity in human skin, Clin. Exp. Immunol., 1997, 107, 585–588 CrossRef CAS.
- H. Miyauchi and T. Horio, Ultraviolet B-induced local immunosuppression of contact hypersensitivity is modulated by ultraviolet irradiation and hapten application, J. Invest. Dermatol., 1995, 104, 364–369 CrossRef CAS.
- H. Miyauchi-Hashimoto and T. Horio, Suppressive effect of ultraviolet B radiation on contact sensitization in mice. II. Systemic immunosuppression is modulated by ultraviolet irradiation and hapten application, Photodermatol. Photoimmunol. Photomed., 1996, 12, 137–144 CAS.
- P. S. Friedmann, The immunology of allergic contact dermatitis: the DNCB story, Adv. Dermatol., 1990, 5, 175–195 Search PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2005 |
