Growth and fragmentation of silver nanoparticles in their synthesis with a fs laser and CW light by photo-sensitization with benzophenone†
Received
27th July 2004
, Accepted 2nd September 2004
First published on 20th September 2004
Abstract
The photo-sensitization synthetic technique of making silver nanoparticles using benzophenone is studied using both a laser and a mercury lamp as light sources. The power and irradiation time dependence of the synthesized nanoparticle absorption spectra and their size distribution [as determined by transmission electron microscopy (TEM)] are studied in each method and compared. In the laser synthesis, as either the laser power or the irradiation time increases, the intensity of the surface plasmon resonance absorption at 400 nm is found to increase linearly first, followed by a reduction of the red edge of the plasmon resonance absorption band. The TEM results showed that in the laser synthesis low powers and short irradiation times produce nanoparticles around 20 nm in diameter. Increasing the power or irradiation time produces a second population of nanoparticles with average size of 5 nm in diameter. These small particles are believed to be formed from the surface ablation of the large particles. The surface plasmon absorption band is found to be narrower when the nanoparticles are produced with laser irradiation. Throughout the exposure time with the CW lamp, the plasmon resonance absorption band of the particles formed first grows in intensity, then blue shifts and narrows, and finally red shifts while decreasing in intensity. The TEM results for lamp samples showed particle formation and growth, followed by small nanoparticle formation. The above results are discussed in terms of a mechanism in which, the excited benzophenone forms the ketal radical, which reduces Ag+ in solution and on the Ag nanoparticle surface. As the time of irradiation or the light energy increases the benzophenone is consumed, which is found to be the limiting reagent. This stops the formation of the normal large nanoparticles while their photo-ablation continues to make the small particles.
Introduction
With the rising importance of nanoparticles, different synthetic methods are being explored to create nanoparticles in solution,1–4 polymer films,5–7 and glasses.8–10 Noble metal nanoparticles have attracted a lot of attention due the plasmon resonance in the visible region which cause these samples to display brilliant colors.11–17 This plasmon resonance is due to the collective oscillation of electrons,11–15 which is sensitive to size and shape and the surrounding medium.16,17 The plasmon resonance allows size and shape transitions to be monitored simply by following UV/visible absorbance. Silver nanospheres are well known to have a plasmon resonance at 400 nm, with the wavelength depending on the size and shape of the nanoparticles. Using photo-generation of nanoparticles allows nanoparticles to be studied in various stages of formation by defining the start of particle formation, as the time in which the reaction mixture is first exposed to light. A number of synthetic methods of these nanoparticles have been developed,1–10,18–22 but little is understood about the growth process.
Recently, photochemical methods have been used to modify and generate metal nanoparticles. Jin and co-workers23,24 have used light to transform silver spheres into prisms by controlling the exposure time and wavelength. Lasers have been used to directly excite and form metal nanoparticles in solution through excitation of the interband transition,25 or excitation of a polymer.7,26–28 These methods create on-demand generation of metal nanoparticles, or photoresponsive solutions. Benzophenone is a well known photo-sensitizer that can be excited by UV radiation. It then relaxes to its lowest triplet excited state which can abstract a hydrogen atom from solution to form radicals capable of performing photochemistry.29–33 Benzophenone has been employed as a photo-sensitizer to form nanoparticles for a variety of metals.6,18–22 Kapoor and Mukherjee have formed copper nanoparticles with a narrow size distribution using benzophenone and PVP in solution.18 The most detailed study presented to date of preparing metal nanoparticles using benzophenone as a photo-sensitizer was preformed by Kometani et al.19 where laser nanosecond flash photolysis was used to determine the radicals of benzophenone during the formation of silver nanoparticles. However, no studies have been carried out in which the growth of the nanoparticles is followed by transmission electron microscopy (TEM).
Femtosecond lasers have enabled the study of ultrafast processes, and allowed distinction between irradiation and subsequent relaxation processes. Previous research showed the difference between melting gold nanorods using a femtosecond and a nanosecond laser.34,35 Femtosecond laser studies enabled following up the different processes involved in the relaxation of the hot electrons excited into the lattice (by electron-phonon process) and then into the environment (by a phonon–phonon process).36–38 If heating is carried on the same scale as cooling, the nanoparticle can either change its shape by melting or by ablation, resulting in the reduction of size due to the continued influx of energy while the particle contains hot electrons. If heating is faster than electrons have a chance to relax, mainly melting is observed.34,35 Due to the high powers of lasers, ablation of metal nanoparticles has been reported.35,39–43 The energy density is so high that the atoms dissociate from the nanoparticle and generate small particles in solution that can be capped by the stabilizer present in the solution.
The present study examines the optical absorption and TEM of particles formed using 266 nm femtosecond laser and mercury lamp irradiation of solutions containing Ag+ and benzophenone as a photo-sensitizer to form silver nanoparticles in aqueous solution. Initially, linear growth of the optical absorbance of the surface plasmon band was observed. This is followed by sub-linear growth of the surface plasmon absorption as a function of time. TEM analysis revealed that the nanoparticle growth is determined by the irradiation conditions and the stabilizer, followed by ablation or aggregation at longer times of irradiation or higher laser powers for laser growth and with longer times of irradiation for the UV lamp irradiation. Nanoparticle formation and ablation compete to form nanoparticles in solution, thus generating different kinetics as time progresses due to the increase in importance of the ablation process. Aggregation is also found to compete with particle formation after benzophenone has been exhausted.
Experimental
The solution used for irradiation contained benzophenone, stabilizer and metal salt. The femtosecond laser irradiation experiments used solution A1 with 3.3 × 10−5 M benzophenone (BP)
(stock solution of benzophenone is dissolved in isopropyl alcohol), 0.3 M isopropyl alcohol (IPA)
(total in solution), and 6.0 × 10−4 M hexadecyltrimethylammonium bromide (CTAB) acting as the stabilizer in water. The solution for mercury lamp irradiation experiments used solution A2 with 3.0 × 10−3 M benzophenone, 0.3 M isopropyl alcohol, and 1 mL per 100 mL solution of Ludox (Aldrich AS-30) dissolved in water. Both systems used the same solution B containing 0.0043 M AgNO3 in water. The final solution contained 2.1 mL of A and 0.9 mL of B. Laser samples were irradiated with a 100 femtosecond laser pulse from an amplified Ti:sapphire laser system (Clark-MXR CPA 1000). The third harmonic at 265 nm (5 µJ maximum power used) of the 795 nm fundamental was used for irradiation experiments. The sample was placed in a quartz 2 mm cylindrical cuvette, and the sample was spun during irradiation. At higher laser powers a ring of silver metal was observed on the inside surface of the cuvette after irradiation, which could be removed with aqua regia. A mercury lamp was used for irradiation in the lamp studies. Absorption spectra were measured on a Shimadzu UV-3103-PC spectrophotometer. For the TEM measurements, a drop of solution was allowed to dry overnight on a carbon coated copper TEM grid (Ted Pella). The samples were imaged at 100kV in the JOEL JEM-100C transmission electron microscope (TEM) to determine the size distributions. Image J (NIH)44 was used to determine the size of each nanoparticle. This program enables thresholding images and size determination from images, with many plugins available for image analysis. The watershed plug-in was used occasionally to separate closely spaced nanoparticles prior to analysis. Histograms of the average diameter (major axis and minor axis averaged) were obtained and fit to Gaussian distributions by Origin 7 SR2 (OriginLab Co.) for three separate spots on each TEM grid and then the fits were averaged for samples from the femtosecond laser. At least 150 particles were counted for each sample, with a thousand particles counted in some cases.
In the limiting reagent experiments, solutions are initially made up the same as above. After 60 min of irradiation, a small amount of the solution was removed from the quartz cuvette, the amount given below was added, and then the cuvette was refilled with the removed solution (to around 600 µL) The same cuvette was used for all experiments to ensure constant volume. The solutions added after 60 min of irradiation are: 100 µL of 0.0043 M AgNO3, 36 µL of IPA and 36 µL of 0.093 M BP in IPA. The vials were then shaken, and the spectra were recorded to observe changes due solely to addition of new solutions. Irradiation was then continued with the femtosecond laser system as described above.
Results and discussion
A. Laser irradiation
Initially, upon irradiation with UV laser light the solution with benzophenone and silver salt develops a faint yellow color, indicating the appearance of silver nanoparticles. As irradiation continues, the solution continues to darken in color indicating the formation of more nanoparticles as the irradiation is continued. Fig. 1 shows the absorbance of the silver/BP solution after various irradiation times with a UV femtosecond laser. This shows an increase in intensity of the plasmon resonance for silver spheres at 400 nm16,17 with increasing laser irradiation. The inset in Fig. 1 shows the spectra normalized to the maximum absorbance to amplify the changes in the shape of the plasmon resonance absorption band. The shape of the plasmon resonance shifts as irradiation time increases, with the relative contribution from the red edge of the plasmon resonance decreasing. The growth of the optical absorbance from the nanoparticles initially displays linearity as shown in Fig. 1b just as described by Kometani et al.19 However, after the first hour of irradiation, the absorbance intensity levels off and seems to decrease at longer time while the spectral shape remains constant (as observed in the inset of Fig. 1) indicating a decrease in the growth rate.
 |
| | Fig. 1 (a) Optical absorbance of silver/benzophenone solution irradiated with 5 µJ, 265 nm 100 femtosecond laser irradiation for various times as indicated. Inset: Absorbance normalized at the peak intensity to observe changes in shape of optical absorbance as a function of irradiation time. (b) Absorbance of sample at 400 nm plotted as a function of the time of irradiation. (c) Absorbance of samples irradiated with 5 µJ, 265 nm femtosecond laser irradiation at 400 nm for 60 min, followed by addition of AgNO3, IPA or BP (dissolved in IPA) and continued irradiation. | |
This decrease in the growth rate was explored to determine the limiting reagent of the nanoparticles formation reaction. This limiting reagent is either benzophenone, the silver salt, the alcohol or the capping material. The sample was irradiated for 60 minutes and then a small volume of a solution was added and the absorption was measured for another hour to determine the kinetics of growth as shown in Fig. 1c. The addition of silver salt to the solution caused a growth rate as observed in Fig. 1b with no additional silver salt, implying that silver ions are not the limiting reagent. Addition of IPA did not change the kinetics, thus this small amount of alcohol did not affect the growth of the nanoparticles. Adding larger volumes of IPA to the solution induced aggregation by destabilizing the nanoparticles due to the presence of a shifting phase equilibra in this water/alcohol system. Adding excess CTAB was able to reduce the effect of additional alcohol by increasing the stabilizing ability of the solution. Increasing the amount of benzophenone in the solution after 60 minutes of irradiation increased the rate of formation of the nanoparticles. These results suggest that benzophenone is the limiting reagent in this synthesis.
We studied the effect of increasing laser power on the absorption spectrum and the size distribution of the silver nanoparticles. Fig. 2 shows the optical absorbance spectra of the nanoparticles formed at three different powers irradiated for an hour with normalized spectra shown in the inset to observe the changes in shape of the optical spectra. The surface plasmon resonance absorption band narrows and the contribution from the red edge are reduced as the power is increased. This shows that long irradiation times and high laser powers have similar effects on the sample. Higher power produces particles with optical absorbance bands much narrower than those observed from the lamp samples. For the same irradiation time, higher powers generate more nanoparticles as observed by their higher optical absorbance. The higher powers also generate more absorption at 400 nm due to selective formation of the smaller nanoparticles as observed in the normalized spectra. TEM is used to follow the formation of nanoparticles at these laser powers.
 |
| | Fig. 2 Optical absorbance of silver/benzophenone solutions irradiated with different powers in the femtosecond laser. Inset: the same spectra normalized to observe changes in shape. | |
The size of the nanoparticles is a function of laser energy and irradiation time. TEM was used determine the shape of the observed absorption intensity changes with increasing laser power. The size distributions can be seen in Fig. 3 with the frequency of each size plotted against the average diameter for different irradiation powers. The statistics for each sample with the center, width, percentage of the width and percentage of large and small spheres are presented in Table 1. As the laser energy increases the population of the small spheres increases and ultimately dominates the population. Thus, the shift in the optical absorption band can be understood by the change in the importance of the mechanism of formation of large nanoparticles versus their ablation at high powers of irradiation. At longer times of irradiation, the amount of benzophenone decreases leading to a decrease in the rate of growth of the large nanoparticles. Meanwhile, as more and more large nanoparticles are formed with time, the rate of their interaction with the laser increases, therefore, the rate of their ablation increases. Thus with increasing irradiation time, the larger nanoparticles decrease in population while that of the small ones increase, as observed.
Table 1 Laser irradiation: energy (0.5, 2.3 and 5 µJ) and irradiation time (5, 60 and 160 min) dependence of the average and standard deviation from 3 TEM spots fitted with position of the two centers (xc1, xc2), the widths (w1, w2), and the percent widths (% width1, % width2), of the small (subscript 1) and large nanoparticle (subscript 2) distributions obtained from Gaussian fits to size distribution data for samples from Fig. 2
| |
0.5 µJ, 60 min |
2.3 µJ, 5 min |
2.3 µJ, 60 min |
5 µJ, 60 min |
5 µJ, 160 min |
| Avg |
Std dev |
Avg |
Std dev |
Avg |
Std dev |
Avg |
Std dev |
Avg |
Std dev |
| xc1 |
|
|
4.3 |
0.2 |
4.2 |
0.6 |
4.0 |
0.5 |
4.2 |
0.7 |
| w1 |
|
|
1.4 |
0.3 |
2.1 |
0.4 |
2.0 |
1.1 |
2.2 |
1.2 |
| % width1 |
|
|
32.9 |
5.1 |
49.8 |
5.3 |
52.7 |
32.6 |
58.8 |
41.5 |
| xc2 |
21.1 |
2.2 |
19.1 |
2.3 |
17.9 |
2.2 |
13.2 |
2.8 |
29.7 |
20.2 |
| w2 |
12.6 |
4.2 |
12.0 |
7.9 |
15.2 |
6.0 |
13.4 |
4.8 |
12.0 |
9.1 |
| % width2 |
58.9 |
13.7 |
60.5 |
32.9 |
88.1 |
46.4 |
99.6 |
13.4 |
73.8 |
67.1 |
| % small |
0.0 |
|
25.2 |
|
69.6 |
|
75.4 |
|
80.8 |
|
| % large |
100.0 |
|
74.8 |
30.0 |
30.4 |
11.3 |
24.6 |
10.4 |
19.2 |
31.3 |
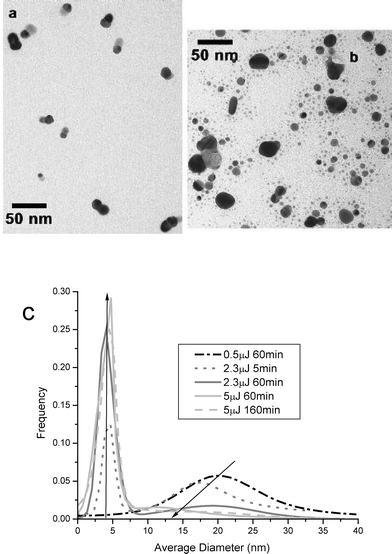 |
| | Fig. 3 (a) TEM image of 0.5 µJ, 60 min femtosecond laser irradiation. (b) TEM image of 5.0 µJ, 60 min femtosecond laser irradiation. (c) Gaussian fits to size distribution data for samples from Fig. 2, femtosecond laser, CTAB stabilizer, at various powers. | |
The size of the nanoparticles remains relatively constant for the smaller nanoparticles at ≈5 nm in diameter. The dominating process at the time of formation determines the sizes generated. The larger nanoparticles are formed first by growth mechanisms, with the size determined by the local concentrations of benzophenone, Ag+, and the stabilizer. The percentage of large spheres in the sample decreases with increasing laser energy, supporting the mechanism of their ablation to form the smaller nanoparticles, as more energy is input to the system. The smaller nanoparticles are proposed to be generated by photo-ablation events of atomic and small clusters from the large particles followed by condensation to form small nanoparticles. Higher powers increase the rate of the ablation events, thus generating more small nanoparticles. At long irradiation time, the loss of the capping material around the nanoparticles (due to the ablation processes, photodecomposition, and increasing surface area) could lead to aggregation and precipitation as reflected by the decline in the optical absorbance observed in Fig. 1b.
B. CW lamp irradiation
In the mercury lamp experiments, different results were obtained from those obtained with the femtosecond laser irradiation as seen in Fig. 4. The absorption band of the lamp samples is broader than that observed in the laser synthetic method and has a long tail to the red of the plasmon resonance band maximum. The broad distribution is not due to the presence of Ludox in the lamp synthetic method. A control experiment was performed on samples with Ludox using femtosecond laser irradiation. The absorption spectrum in this control sample was narrower than that of the lamp irradiated samples and the sample from the femtosecond laser experiments discussed previously. In the time dependence experiments, the optical absorbance first grows in intensity and blue shifts, followed by a red shift with decreasing absorbance intensity at longer times. This data suggests separate competing processes are involved. First nanoparticles might grow by Oswald ripening processes, followed by aggregation and precipitation. Lamp irradiation is useful as the source is easily attainable, generating nanoparticles with a changing optical absorbance spectrum that is observed with continuous irradiation. After two hours of exposure, a new population appears which absorbs at longer wavelengths, characteristic of aggregated nanoparticles. The decrease in the absorption with long irradiation times suggests precipitation of the aggregate. This leaves behind a population of newly formed small nanoparticles.
 |
| | Fig. 4 (a) Optical absorbance of silver/benzophenone solution with mercury lamp irradiation at times indicated. Inset: Absorbance normalized at the peak intensity to observe changes in shape of optical absorbance as a function of irradiation time. (b) TEM image of silver/benzophenone solution after mercury lamp irradiation, 45 min. (c) Gaussian fits to size distribution data for the same samples, mercury lamp, Ludox stabilizer, at times indicated. | |
The TEM results of the produced particles in the mercury lamp irradiation are presented in Fig. 4c and Table 2. The size distribution is broad at short irradiation times, but becomes smaller after 45 minutes of irradiation with an average diameter of 10 nm nanoparticles generated. After two hours of irradiation the relative population of the small nanoparticles increases, with a broad distribution of nanoparticles of larger diameter formed due to aggregation. A decrease in the total absorption is also observed after two hours suggesting some nanoparticles have already precipitated. Thus, complex kinetics of formation generate different nanoparticle sizes initially. First growth by Oswald ripening, then aggregation and finally precipitation lead first to a broad distribution, and then precipitation of the larger particles leaving the small particles in solution. Lamp irradiation generates nanoparticles using benzophenone sensitization and Ludox as a stabilizer. The polydispersity observed is large in all samples generated with lamp irradiation.
Table 2 Mercury lamp irradiation: irradiation time dependence of the position of the two centers (xc1, xc2), the widths (w1, w2), and the percent widths (% width), of the small (subscript 1)and large nanoparticle (subscript 2) distributions obtained from Gaussian fits to size distribution data for samples from Fig. 4
| |
Small |
Large |
|
|
| Time(min) |
Xc1 |
W1 |
% width |
Xc2 |
W2 |
% width |
% small |
% large |
| 0.5 |
7.8 |
4.3 |
54.9 |
15.3 |
6.5 |
42.2 |
29.1 |
70.9 |
| 1 |
3.1 |
3.2 |
101.2 |
15.8 |
10.4 |
66.1 |
82.0 |
18.0 |
| 5 |
5.8 |
7.8 |
134.2 |
14.1 |
5.7 |
40.7 |
58.9 |
41.1 |
| 20 |
|
|
|
9.6 |
9.4 |
98.1 |
0.0 |
100.0 |
| 45 |
|
|
|
10.9 |
6.0 |
55.4 |
0.0 |
100.0 |
| 120 |
3.0 |
3.6 |
118.4 |
13.4 |
13.0 |
97.1 |
57.2 |
42.8 |
C. Proposed growth mechanism
The above results can be discussed by use of the following mechanism for particle growth. The benzophenone ketyl radical (BPK), is formed from the abstraction reaction of benzophenone in its triplet state of a hydrogen atom from the solvent, isopropyl alcohol (ROH). This also generates the alcohol radical. The silver atoms are then reduced by the benzophenone ketyl radical to form nuclei,19 which can then grow by reducing more silver ions on the surface of the nanoparticles or by condensation of atomic silver on the silver nuclei to form (Ag)L, large silver nanoparticles. Capping is occurring and limits the size of the (Ag)L. At the same time the absorption of photons by the strong absorbing (Ag)L is continuously occurring leading to the ablation of surface silver atoms. This leaves behind the (Ag)S, small silver nanoparticles, which is observed in the TEM results. The chemical reactions describing this mechanism can be written as:| |  | (1) |
| |  | (2) |
| |  | (3) |
| |  | (4) |
| |  | (5) |
| |  | (6) |
This mechanism is able to describe the formation of silver atoms, as well as the growth of the nanoparticles. At long irradiation times, the benzophenone, whose initial concentration is limited by its solubility in the solvent, is consumed. This stops the formation of the larger nanoparticles by the direct mechanism. However, larger nanoparticles can still be formed by an Oswald ripening from the small particles by aggregation to shift the absorption to longer wavelengths. After the benzophenone is consumed, the laser ablation processes are at their maximum rate as the concentration of the large nanoparticles is at its maximum. Thus the net concentration of the large particles slows down while the ablation rate is at its maximum. This leads to an increase in the relative amounts of the small nanoparticles present in solution.
Summary
The TEM results presented here demonstrate the growth mechanism is followed by ablation for photosensitized metal nanoparticles using benzophenone. Changes are observed in the optical spectra which can be used to follow the generation of nanoparticles, but TEM is required to show the increasing importance of the ablation mechanism in the laser synthesis as the energy or time of irradiation increases. Nanoparticle growth was observed with mercury lamp and femtosecond laser irradiation with, as stabilizers determining the size of nanoparticles generated, Ludox and CTAB, respectively. Increasing irradiation time in the lamp synthesis leads to a narrowing of the plasmon resonance band, which could be correlated to a narrowing of the particle distribution by TEM for both laser and lamp irradiation due to Oswald ripening mechanisms. Lamp and laser irradiation both generated aggregated nanoparticles after long irradiation times. Laser irradiation also ablated the samples at high laser powers. The systematic changing of the laser power and irradiation time showed that growth of large nanoparticles takes place before benzophenone was consumed, while ablation in the laser experiments is taking place throughout the exposure time. The ablation rate increases as the concentration of the large nanoparticles increases. At longer times or high power, the benzophenone concentration decreases along with the rate of formation of the large particles while the rate of ablation is at its maximum.
Acknowledgements
The authors wish to acknowledge the financial support of the Nation Science Foundation, Division of Material Science grant No. 0138391.
References
- Y. Y. Yu, S. S. Chang, C. L. Lee and C. R. C. Wang, Gold nanorods: Electrochemical synthesis and optical properties, J. Phys. Chem. B, 1997, 101(34), 6661–6664 CrossRef CAS.
- J. Turkevich, P. C. Stevenson and J. Hillier, A study of the nucleation and growth processes in the synthesis of colloidal gold, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- C. Petit, P. Lixon and M. P. Pileni, In situ synthesis of silver nanocluster in aot reverse micelles, J. Phys. Chem., 1993, 97(49), 12974–12983 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Seeding growth for size control of 5–40 nm diameter gold nanoparticles, Langmuir, 2001, 17(22), 6782–6786 CrossRef CAS.
- O. L. A. Monti, J. T. Fourkas and D. J. Nesbitt, Diffraction-limited photogeneration and characterization of silver nanoparticles, J. Phys. Chem. B, 2004, 108(5), 1604–1612 CrossRef CAS.
- A. S. Korchev, M. J. Bozack, B. L. Slaten and G. Mills, Polymer-initiated photogeneration of silver nanoparticles in speek/pva films: Direct metal photopatterning, J. Am. Chem. Soc., 2004, 126(1), 10–11 CAS.
- S. Weaver, D. Taylor, W. Gale and G. Mills, Photoinitiated reversible formation of small gold crystallites in polymer gels, Langmuir, 1996, 12(20), 4618–4620 CrossRef CAS.
- J. Qiu, X. Jiang, C. Zhu, M. Shirai, J. Si, N. Jiang and K. Hirao, Manipulation of gold nanoparticles inside transparent materials, Angew. Chem., Int. Ed., 2004, 43, 2230–2234 CrossRef CAS.
- X. Jiang, J. Qiu, H. Zeng, C. Zhu and K. Hirao, Laser-controlled dissolution of gold nanoparticles in glass, Chem. Phys. Lett., 2004, 391, 91–94 CrossRef CAS.
- J. Qiu, M. Shirai, T. Nakaya, J. Si, X. Jiang, C. Zhu and K. Hirao, Space-selective precipitation of metal nanoparticles inside glasses, Appl. Phys. Lett., 2002, 81(16), 3040–3042 CrossRef CAS.
-
U. Kreibig and M. Vollmer, Optical Properties of Metal Clusters, Springer, Berlin, 1995 Search PubMed.
- P. Mulvaney, Surface plasmon spectroscopy of nanosized metal particles, Langmuir, 1996, 12, 788–800 CrossRef CAS.
- A. Henglein, Physicochemical properties of small metal particles in solution: “microelectrode” reactions, chemisorption, composite metal particles, and the atom-to-metal transition, J. Phys. Chem., 1993, 97(21), 5457–5471 CrossRef CAS.
- A. Henglein, Small-particle research: Physicochemical properties of extremely small colloidal metal and semiconductor particles, Chem. Rev., 1989, 89(8), 1861–1873 CrossRef CAS.
-
C. F. Bohren and D. R. Huffman, Absorption and scattering of light by small particles, Wiley, New York, 1983 Search PubMed.
- U. Kreibig and C. v. Fragstein, The limitation of electron mean free path in small silver particles, Z. Phys., 1969, 224, 307–323 CAS.
- A. Kawabata and R. Kubo, Electronic properties of fine metallic particles. Ii. Plasmon resonance absorption, J. Phys. Soc. Jpn., 1966, 21(9), 1765–1772 CAS.
- S. Kapoor and T. Mukherjee, Photochemical formation of copper nanoparticles in poly(n-vinylpyrrolidone), Chem. Phys. Lett., 2003, 370(1-2), 83–87 CrossRef CAS.
- N. Kometani, H. Doi, K. Asami and Y. Yonezawa, Laser flash photolysis study of the photochemical formation of colloidal Ag nanoparticles in the presence of benzophenone, Phys. Chem. Chem. Phys., 2002, 4, 5142–5147 RSC.
- S. Kapoor, Preparation, characterization, and surface modification of silver particles, Langmuir, 1998, 14(5), 1021–1025 CrossRef CAS.
- T. Sato, N. Maeda, H. Ohkoshi and Y. Yonezawa, Photochemical formation of colloidal silver in the presence of benzophenone, Bull. Chem. Soc. Jpn., 1994, 67(12), 3165–3171 CAS.
- T. Sato, H. Onaka and Y. Yonezawa, Sensitized photoreduction of silver ions in the presence of acetophenone, J. Photochem. Photobiol. A, 1999, 127(1-3), 83–87 CrossRef CAS.
- R. C. Jin, Y. C. Cao, E. C. Hao, G. S. Metraux, G. C. Schatz and C. A. Mirkin, Controlling anisotropic nanoparticle growth through plasmon excitation, Nature, 2003, 425(6957), 487–490 CrossRef CAS.
- R. C. Jin, Y. W. Cao, C. A. Mirkin, K. L. Kelly, G. C. Schatz and J. G. Zheng, Photoinduced conversion of silver nanospheres to nanoprisms, Science, 2001, 294(5548), 1901–1903 CrossRef CAS.
- F. Mafune and T. Kondow, Formation of small gold clusters in solution by laser excitation of interband transition, Chem. Phys. Lett., 2003, 372, 199–204 CrossRef CAS.
- M. Mandal, S. K. Ghosh, S. Kundu, K. Esumi and T. Pal, UV photoactivation for size and shape controlled synthesis and coalescence of gold nanoparticles in micelles, Langmuir, 2002, 18(21), 7792–7797 CrossRef CAS.
- Y. Zhou, S. H. Yu, C. Y. Wang, X. G. Li, Y. R. Zhu and Z. Y. Chen, A novel ultraviolet irradiation photoreduction technique for the preparation of single-crystal Ag nanorods and Ag dendrites, Adv. Mater., 1999, 11(10), 850–852 CrossRef CAS.
- L. Longenberger and G. Mills, Formation of metal particles in aqueous-solutions by reactions of metal-complexes with polymers, J. Phys. Chem., 1995, 99(2), 475–478 CrossRef CAS.
- J. L. Marignier and B. Hickel, Pulse radiolysis measurements of the solvation rate of benzophenone anion in liquid alcohol: Effect of temperature, J. Phys. Chem., 1984, 88(22), 5375–5379 CrossRef CAS.
- K. S. Peters and J. Lee, Picosecond dynamics of the photoreduction of benzophenone by dabco, J. Phys. Chem., 1993, 97(15), 3761–3764 CrossRef CAS.
- H. Miyasaka, T. Nagata, M. Kiri and N. Mataga, Femtosecond–picosecond laser photolysis studies on reduction process of excited benzophenone with n-methyldiphenylamine in acetonitrile solution, J. Phys. Chem., 1992, 96(20), 8060–8065 CrossRef CAS.
- M. Hoshino, S. Aral, M. Imamura, K. Ikehara and Y. Hama, Mechanism of benzophenone ketyl radical formation in acid alcohols studied by pulse-radiolysis and rigid-matrix techniques, J. Phys. Chem., 1980, 84(20), 2576–2579 CrossRef CAS.
- H. Mural, M. Jinguji and K. Obl, Activation energy of hydrogen atom abstraction by triplet benzophenone at low temperature, J. Phys. Chem., 1978, 82(1), 38–40 CrossRef.
- S. Link, C. Burda, B. Nikoobakht and M. A. El-Sayed, Laser-induced shape changes of colloidal gold nanorods using femtosecond and nanosecond laser pulses, J. Phys. Chem. B, 2000, 104(26), 6152–6163 CrossRef CAS.
- S. Link, C. Burda, M. B. Mohamed, B. Nikoobakht and M. A. El-Sayed, Laser photothermal melting and fragmentation of gold nanorods: Energy and laser pulse-width dependence, J. Phys. Chem. A, 1999, 103(9), 1165–1170 CrossRef CAS.
- M. Hu and G. V. Hartland, Heat dissipation for au particles in aqueous solution: Relaxation time versus size, J. Phys. Chem. B, 2002, 106(28), 7029–7033 CrossRef CAS.
- C. Voisin, N. Del Fatti, D. Christofilos and F. Vallee, Ultrafast electron dynamics and optical nonlinearities in metal nanoparticles, J. Phys. Chem. B, 2001, 105(12), 2264–2280 CrossRef CAS.
- S. Link, A. Furube, M. B. Mohamed, T. Asahi, H. Masuhara and M. A. El-Sayed, Hot electron relaxation dynamics of gold nanoparticles embedded in MgSO4 powder compared to solution: The effect of the surrounding medium, J. Phys. Chem. B, 2002, 106(5), 945–955 CrossRef CAS.
- H. Kurita, A. Takami and S. Koda, Size reduction of gold particles in aqueous solution by pulsed laser irradiation, Appl. Phys. Lett., 1998, 72(7), 789–791 CrossRef CAS.
- F. Mafune, J. Y. Kohno, Y. Takeda and T. Kondow, Growth of gold clusters into nanoparticles in a solution following laser-induced fragmentation, J. Phys. Chem. B, 2002, 106(34), 8555–8561 CrossRef CAS.
- F. Mafune, J. Kohno, Y. Takeda, T. Kondow and H. Sawabe, Formation and size control of sliver nanoparticles by laser ablation in aqueous solution, J. Phys. Chem. B, 2000, 104(39), 9111–9117 CrossRef CAS.
- A. Takami, H. Kurita and S. Koda, Laser-induced size reduction of noble metal particles, J. Phys. Chem. B, 1999, 103(8), 1226–1232 CrossRef CAS.
- P. V. Kamat, M. Flumiani and G. V. Hartland, Picosecond dynamics of silver nanoclusters. Photoejection of electrons and fragmentation, J. Phys. Chem. B, 1998, 102(17), 3123–3128 CrossRef CAS.
- ImageJ is a public domain Java image processing program inspired by NIH Image. Available at http://rsb.info.nih.gov/ij/, with the source code freely available. The Watershead plugin and other plugins are also available. Wayne Rosband, Bethesda, MD, 1997–2004.
Footnote |
| † Dedicated to Professor Hiroshi Masuhara on the occasion of his 60th birthday. |
|
| This journal is © The Royal Society of Chemistry and Owner Societies 2005 |
Click here to see how this site uses Cookies. View our privacy policy here.